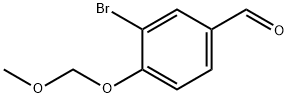
3-Bromo-4-(methoxymethoxy)benzaldehyde synthesis
- Product Name:3-Bromo-4-(methoxymethoxy)benzaldehyde
- CAS Number:162269-90-1
- Molecular formula:C9H9BrO3
- Molecular Weight:245.07
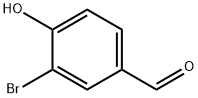
2973-78-6
214 suppliers
$14.00/5g
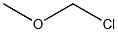
107-30-2
1 suppliers
$110.00/2.5g

162269-90-1
13 suppliers
inquiry
Yield:162269-90-1 100%
Reaction Conditions:
Stage #1: 3-bromo-4-hydroxybenzylaldehydewith sodium hydride in tetrahydrofuran;N,N-dimethyl-formamide at 20; for 0.216667 h;
Stage #2: chloromethyl methyl ether in tetrahydrofuran;N,N-dimethyl-formamide at 20; for 1 h;
Steps:
2.b
(b) Preparation of 3-Bromo-4-methoxymethoxybenzaldehyde: 9 g (44.7 mmol) of 3-bromo-4-hydroxy-benzaldehyde, 30 ml of THF and 30 ml of DMF were introduced into a 250 ml round-bottomed flask under a nitrogen atmosphere. 1.48 g (49.2 mmol) of 80% sodium hydride were added portionwise, the mixture was stirred for thirty minutes at room temperature and 4.32 g (52.7 mmol) of methoxymethyl chloride were added dropwise. The reaction medium was stirred for one hour at room temperature, acidified with 1N hydrochloric acid and extracted with ethyl acetate. The organic phase was separated out after settling had taken place, washed with water to neutral pH and dried over magnesium sulfate, and the solvents were evaporated off. 11.5 g (100%) of an orange-colored oil were collected.
References:
US6632963,2003,B1 Location in patent:Page/Page column 13-14

13057-17-5
168 suppliers
$26.00/5g

2973-78-6
214 suppliers
$14.00/5g

162269-90-1
13 suppliers
inquiry

123-08-0
885 suppliers
$5.00/10g

162269-90-1
13 suppliers
inquiry
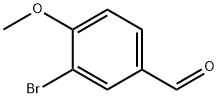
34841-06-0
235 suppliers
$8.00/5g

162269-90-1
13 suppliers
inquiry
