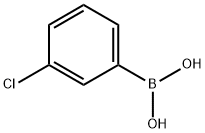
3-Chlorophenylboronic acid synthesis
- Product Name:3-Chlorophenylboronic acid
- CAS Number:63503-60-6
- Molecular formula:C6H6BClO2
- Molecular Weight:156.37
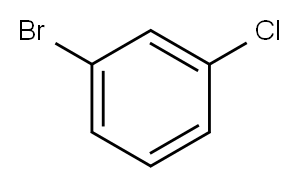
108-37-2
356 suppliers
$6.00/10g

63503-60-6
428 suppliers
$9.00/1g
Yield:63503-60-6 57%
Reaction Conditions:
Stage #1:1-bromo-3-chlorobenzene with n-butyllithium in tetrahydrofuran;hexaneInert atmosphere;
Stage #2: with Trimethyl borate in tetrahydrofuran;hexaneInert atmosphere;
Steps:
General procedure for the synthesis of arylboronic acids
General procedure: Under an argon atmosphere a solution of the appropriate bromobenzene (1 equivalent) dissolved in anhydrous THF (approximately 30 mL per mmol bromobenzene) is cooled to -78 °C using a nitrogen-ethanol-bath. A solution of 2.3 equivalents of n-butyllithium in hexane is added drop wise keeping the temperature below -78 °C. After completion the mixture is stirred for one hour at this temperature. Then 1.5 equivalents of trimethyl borate are added slowly and the reaction mixture is stirred at -78 °C for another hour. The cooling bath is then removed, the reaction mixture is stirred until room temperature is reached and quenched with a saturated solution of ammonium chloride. THF and the major part of the water is removed under reduced pressure, the residue is laced with 3M hydrochloric acid until a pH of 3 is reached. After extraction with DCM (3 x) the organic phases are collected, washed with brine, dried over sodium sulphate and filtered. DCM is removed under reduced pressure, the resulting solid is washed first with ice cold water and then with PE and dried.
References:
Obermoser, Victoria;Mauersberger, Robert;Schuster, Daniela;Czifersky, Monika;Lipova, Marina;Siegl, Monika;Kintscher, Ulrich;Gust, Ronald [European Journal of Medicinal Chemistry,2017,vol. 126,p. 590 - 603] Location in patent:supporting information

108-37-2
356 suppliers
$6.00/10g
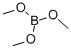
121-43-7
345 suppliers
$13.00/25mL

63503-60-6
428 suppliers
$9.00/1g

108-37-2
356 suppliers
$6.00/10g
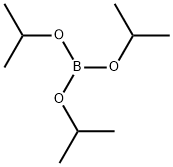
5419-55-6
369 suppliers
$12.00/5g

63503-60-6
428 suppliers
$9.00/1g

108-37-2
356 suppliers
$6.00/10g
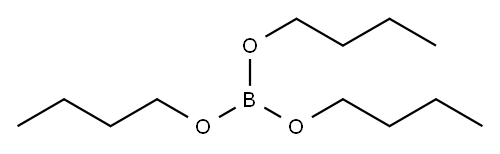
688-74-4
310 suppliers
$10.40/25mL

63503-60-6
428 suppliers
$9.00/1g

108-37-2
356 suppliers
$6.00/10g
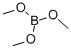
121-43-7
345 suppliers
$13.00/25mL

63503-60-6
428 suppliers
$9.00/1g
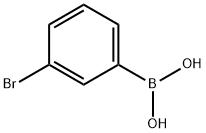
89598-96-9
351 suppliers
$9.00/1g