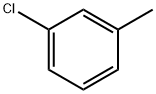
3-Chlorotoluene synthesis
- Product Name:3-Chlorotoluene
- CAS Number:108-41-8
- Molecular formula:C7H7Cl
- Molecular Weight:126.58
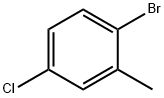
14495-51-3
249 suppliers
$10.00/5g

108-41-8
261 suppliers
$19.00/10g
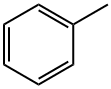
108-88-3
7 suppliers
$21.80/5 mL
Yield:108-41-8 21 %Chromat. ,108-88-3 40 %Chromat.
Reaction Conditions:
with Pd-γ-Fe2O3;potassium tert-butylate in isopropyl alcohol at 70; for 12 h;Inert atmosphere;Sealed tube;
Steps:
Dehalogenation of 2-Bromo-1,3,5-trimethylbenzene; 1,3,5-Trimethylbenzene (6a); Typical Procedure for the Dehalogenation Reaction
General procedure: To a dry glass reaction tube purged with argon and equipped with a magnetic stir bar were added 2-bromo-1,3,5-trimethylbenzene (5a; 40 μL, 0.26 mmol), Pd-γ-Fe2O3 (25 mg, 5 mol% Pd), NaOt-Bu (31 mg, 0.33 mmol), and i-PrOH (1 mL) and the sealed tube was heated at 70 °C for 12 h. GC-MS yield of 6a: 100%, based on naphthalene as standard.
References:
Ajdačić, Vladimir;Nikolić, Andrea;Simić, Stefan;Manojlović, Dragan;Stojanović, Zoran;Nikodinovic-Runic, Jasmina;Opsenica, Igor M. [Synthesis,2018,vol. 50,# 1,art. no. SS-2017-T0452-OP,p. 119 - 126]

108-88-3
7 suppliers
$21.80/5 mL

108-41-8
261 suppliers
$19.00/10g
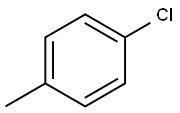
106-43-4
332 suppliers
$14.00/5g
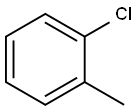
95-49-8
328 suppliers
$10.00/5g
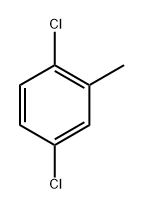
19398-61-9
115 suppliers
$10.00/5 g

108-41-8
261 suppliers
$19.00/10g

95-49-8
328 suppliers
$10.00/5g

108-88-3
7 suppliers
$21.80/5 mL