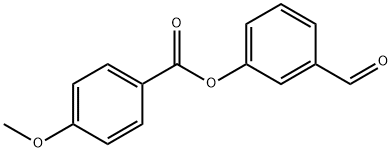
3-FORMYLPHENYL 4-METHOXYBENZOATE synthesis
- Product Name:3-FORMYLPHENYL 4-METHOXYBENZOATE
- CAS Number:146952-27-4
- Molecular formula:C15H12O4
- Molecular Weight:256.25
Yield:146952-27-4 78.6%
Reaction Conditions:
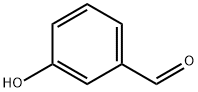
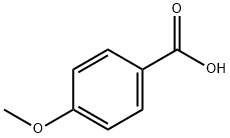
Stage #1: meta-hydroxybenzaldehyde;4-methoxybenzoic acidwith dmap in N,N-dimethyl-formamide at 20; for 0.25 h;
Stage #2: with dicyclohexyl-carbodiimide in N,N-dimethyl-formamide at 20;
Steps:
4.3.5 General procedure for the synthesis of aldehyde scaffolds 7a-7n and 8a-8n
General procedure: 3-Hydroxybenzaldehyde (for 7a-7n) or 4-hydroxybenzaldehyde (for 8a-8n) (1.0g, 8.19mmol), DMAP (0.05g, 0.4mmol) and the corresponding 4-alkoxy benzoic acid (8.19mmol) were dissolved in DMF and stirred at room temperature for 15min. Then DCC (2.0g, 9.83mmol) was added drop wise to the reaction mixture. After completion of reaction, the reaction mixture was diluted with ethyl acetate and filtered through celite. The filtrate was washed with water and brine solution. The organic portion was dried over sodium sulfate and concentrated under reduced pressure. The crude was then purified by column chromatography (silica gel 100-200 mesh) using ethyl acetate in hexanes to obtain the product. 4.3.6 Synthesis of 3-formylphenyl 4-methoxybenzoate (7a) Yield: 1.65g, 78.6%, white solid, m.p: 78-80°C, 1H NMR (400MHz, CDCl3): δ 3.91 (s, 3H), 7.01 (dd, J=2.0, 6.8Hz, 2ArH), 7.48-7.51 (m, 1ArH), 7.60 (t, J=7.6Hz, 1ArH), 7.75 (d, J=2.0Hz, 1ArH), 7.79-7.81 (m, 1ArH), 8.17 (dd, J=2.0, 6.8Hz, 2ArH), 10.04 (s, 1H CHO). HRMS (ESI+): m/z calculated for C15H13O4 [M+H]+: 257.0814; found: 257.0815.
References:
Datta, Dhrubajyoti;Debnath, Joy;Franzblau, Scott G.;Ghosh, Kalyan Sundar;Hari, Natarajan;Ma, Rui;Rana, Shiwani;Velappan, Anand Babu [Bioorganic Chemistry,2020,vol. 103,art. no. 104170]
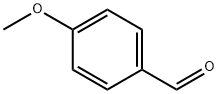
123-11-5
833 suppliers
$10.00/10g

146952-27-4
6 suppliers
inquiry

123-08-0
883 suppliers
$5.00/10g

146952-27-4
6 suppliers
inquiry