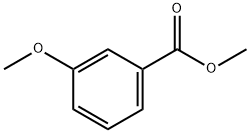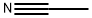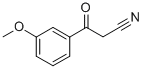
3-Methoxybenzoylacetonitrile synthesis
- Product Name:3-Methoxybenzoylacetonitrile
- CAS Number:21667-60-7
- Molecular formula:C10H9NO2
- Molecular Weight:175.18
Yield: 83%
Reaction Conditions:
Stage #1:acetonitrile with lithium diisopropyl amide in tetrahydrofuran at -78; for 0.833333 h;
Stage #2:methyl 3-methoxybenzoate in tetrahydrofuran for 2.5 h;
Steps:
1a.1
LDA (44.2 ml. of a 2M solution in THF, 88.5 mmol) was added dropwise over 20 minutes to a cold (-78 0C) solution of acetonitrile (2.8 ml_, 53.6 mmol) in THF (63 mL) under an atmosphere of nitrogen. The reaction mixture was stirred for 30 minutes and a solution of methyl 3-methoxybenzoate (7.00 g, 42.1 mmol) in THF (63 mL) was added dropwise over 30 minutes. The reaction mixture was stirred for 2 hours, quenched with a saturated aqueous solution of ammonium chloride (100 mL), allowed to warm to room temperature and diluted with Et2O (300 mL). The aqueous layer was separated and extracted with Et2O (100 mL). The combined organic layers were washed with 1 M HCI (200 mL), brine, dried (MgSO4) and concentrated under reduced pressure. The residue was purified by column chromatography (25-30 % EtOAc in heptane) to afford the title compound as a pale orange solid (6.16 g, 83 % yield). 1H NMR (300 MHz, CDCI3) 7.50-7.42 (3H, m), 7.24-7.20 (1 H, m), 4.10 (2H, s), 3.89 (3H, s).
References:
Location in patent:Page/Page column 27

474843-37-3
0 suppliers
inquiry

21667-60-7
74 suppliers
inquiry

18293-53-3
72 suppliers
$109.00/5g
766-85-8
190 suppliers
$22.00/5g
201230-82-2
1 suppliers
inquiry

21667-60-7
74 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

21667-60-7
74 suppliers
inquiry