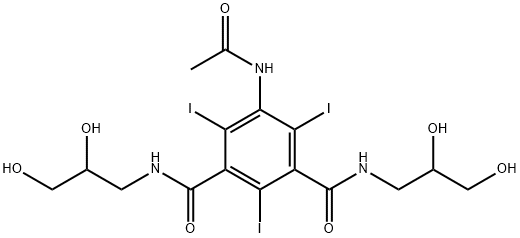
5-(Acetamido)-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide synthesis
- Product Name:5-(Acetamido)-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide
- CAS Number:31127-80-7
- Molecular formula:C16H20I3N3O7
- Molecular Weight:747.06
![1,3-Benzenedicarboxamide, 5-(acetylamino)-N1,N3-bis[2,3-bis(acetyloxy)propyl]-2,4,6-triiodo-](/CAS/20200611/GIF/174416-65-0.gif)
174416-65-0
1 suppliers
inquiry

31127-80-7
222 suppliers
$24.00/5g
Yield:31127-80-7 98.9%
Reaction Conditions:
with sodium hydroxide in methanol;lithium hydroxide monohydrate at 45 - 75; for 8 h;Large scale;
Steps:
1-7 Example 4, Preparation of iohexol intermediate (compound of formula 3)
In the 3000L glass-lined reactor, add 800kg of the compound of formula 1, a mixture of 450kg acetic acid and 1350kg acetic anhydride was slowly added dropwise at a temperature of 50 to 60°C with 6L of concentrated sulfuric acid, and then the temperature was controlled at a temperature of 70 to 80°C and reacted for 3 to 6 hours. After the temperature drops to 40-50°C, the acetic anhydride is recovered under reduced pressure until no liquid is distilled. Add 600kg of methanol under 75°C temperature control, stir and dissolve, transfer to the hydrolysis kettle, add 1400L of water, add about 500kg of 50% sodium hydroxide solution dropwise at 4555°C temperature control, and complete the reaction for 8 hours. Add hydrochloric acid dropwise to adjust the pH to 5-7, lower the temperature at 10-20° C. for 3-6 hours, filter by plate and frame, and dry under reduced pressure to obtain 837.4 kg of the iohexol intermediate with a yield of 98.9% and a purity of 99.496%.
References:
CN114853625,2022,A Location in patent:Paragraph 0029-0046
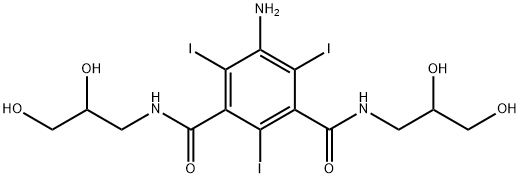
76801-93-9
222 suppliers
$23.00/25g

108-24-7
5 suppliers
$14.00/250ML

64-19-7
1528 suppliers
$10.00/25ML

31127-80-7
222 suppliers
$24.00/5g

76801-93-9
222 suppliers
$23.00/25g

75-36-5
560 suppliers
$17.92/100G

31127-80-7
222 suppliers
$24.00/5g

31122-75-5
14 suppliers
$250.00/2mg
13552-31-3
0 suppliers
inquiry

31127-80-7
222 suppliers
$24.00/5g
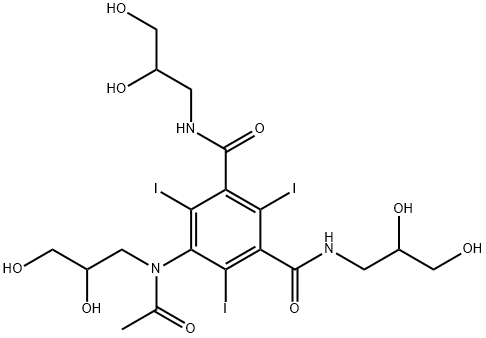
66108-95-0
384 suppliers
$28.00/1g
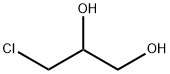
96-24-2
291 suppliers
$11.00/5g

31127-80-7
222 suppliers
$24.00/5g