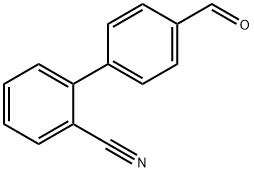
4-(2-Cyanophenyl)benzaldehyde synthesis
- Product Name:4-(2-Cyanophenyl)benzaldehyde
- CAS Number:135689-93-9
- Molecular formula:C14H9NO
- Molecular Weight:207.23
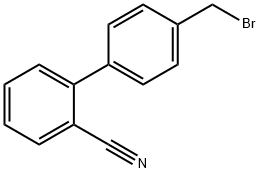
114772-54-2
594 suppliers
$5.00/5g

135689-93-9
66 suppliers
inquiry
Yield:135689-93-9 77.3%
Reaction Conditions:
with hexamethylenetetramine;acetic acid in water;chlorobenzene; pH=7 - 8 at 85 - 93; for 10 h;Product distribution / selectivity;
Steps:
1
Example 1: production of CBAL To monochlorobenzene (1000 g)was added CMBMB (400 g, 1.47 mol), then, water (812 g), hexamethylenetetramine (412 g, 2.94 mol) and acetic acid (618 g, 10.29 mol) were added, then, the solution was stirred at 93°C for 9 hours. The solution was allowed to stand still for 1 hour at 85 to 90°C and phase-separation was conducted. To organic layer was added water (795 g), then, 10% potassium carbonate aqueous solution (684 g) was added to control pH at from 7 to 8. The solution was allowed to stand still for 1 hour, then, phase-separation was conducted. 142 ml of monochlorobenzene was distilled off at 85 to 95°C under a reduced pressure of 22.7 to 33.3 kPa. The solution was stirred at 70°C for 2 hours to cause growth of crystals, then, cooled down to 0 to 5°C at a rate of 10°C/hour and stirred at the same temperature for 5 hors. Filtration is performed, and the resultant crystals were washed with monochlorobenzene (400 g) cooled to about 5°C, and dried at about 60°C to obtain CBAL (235.6 g). The yield was 77.3%. 1H-NMR (400 MHz, CDCl3, dppm), 7.5 (2H, m, Ph), 7.6 (1H, m, Ph), 7.7 (2H, d, Ph), 7.8 (1H, m, Ph), 8.0 (2H, d, Ph),10.1 (1H, s, CHO) IR (KBr) v 2224,1687 cm-1
References:
Sumitomo Chemical Company, Limited EP1666471, 2006, A1 Location in patent:Page/Page column 9
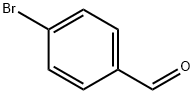
1122-91-4
672 suppliers
$9.00/10g

214360-47-1
112 suppliers
$5.00/100mg

135689-93-9
66 suppliers
inquiry
![4'-(Dibromomethyl)-[1,1'-Biphenyl]-2-Carbonitrile](/CAS/GIF/209911-63-7.gif)
209911-63-7
76 suppliers
inquiry

114772-54-2
594 suppliers
$5.00/5g

135689-93-9
66 suppliers
inquiry
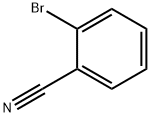
2042-37-7
347 suppliers
$7.00/10g
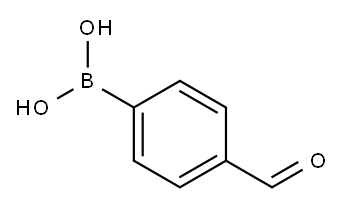
87199-17-5
415 suppliers
$8.00/5g

135689-93-9
66 suppliers
inquiry
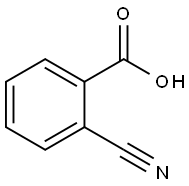
3839-22-3
169 suppliers
$15.00/1g

1122-91-4
672 suppliers
$9.00/10g

135689-93-9
66 suppliers
inquiry