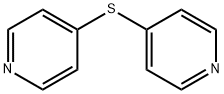
4,4'-DIPYRIDYL SULFIDE synthesis
- Product Name:4,4'-DIPYRIDYL SULFIDE
- CAS Number:37968-97-1
- Molecular formula:C10H8N2S
- Molecular Weight:188.25
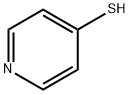
4556-23-4
198 suppliers
$6.00/1g
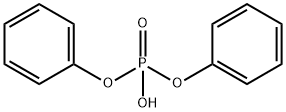
838-85-7
263 suppliers
$9.00/1g
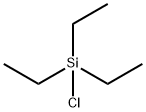
994-30-9
406 suppliers
$12.00/5g
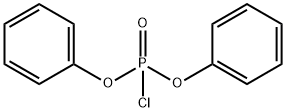
2524-64-3
386 suppliers
$14.00/5g

37968-97-1
62 suppliers
$23.00/100mg
Yield:37968-97-1 71.6%
Reaction Conditions:
with dmap;triethylamine in dichloromethane;acetonitrile
Steps:
F.4 EXAMPLE F-4
EXAMPLE F-4 R1 =1-(triethylsilyloxy)ethyl, R2 =methyl, OR6 =OH→Cl→SHet, R7 =p-methoxybenzyl, Het=4-pyridyl. Alcohol (a) (800 mg; 1.68 millimoles) is esterified with diphenyl chlorophosphate (0.42 ml; 1.2 equivalents), 4-(dimethylamino)pyridine (40 mg; 0.2 equivalents), and triethylamine (0.28 ml; 1.2 equivalents) in dichloromethane (10 ml) at -60° C. for 30 minutes and recovered its silyl with triethylsilyl chloride (0.75 ml; 3.9 equivalents) for 1 hour as in Example B-10 to give starting 2-chloride of (a). To a solution of this 2-chloride of (a) in acetonitrile (10 ml) are added 4-mercaptopyridine (280 mg; 1.5 equivalents) and triethylamine (0.35 ml; 1.5 equivalents). After 1 hour at -20° C., the reaction mixture is diluted with dichloromethane and poured into aqueous sodium hydrogen carbonate. The organic layer is separated, washed with aqueous ammonium chloride and water, dried (MgSO4), and concentrated in vacuum. The residue is purified by chromatography over silica gel (60 g) using toluene-ethyl acetate (1:2) to give 4-pyridyl sulfide (b) (680 mg). Yield: 71.6%. NMR (EM-390, CDCl3)δ: 0.52-0.68 (6H, m), 0.85-1.00 (9H, m), 1.15 (3H, d, J=8.7 Hz), 1.23 (3H, d, J=6 Hz), 3.18 (1H, dd, J=6.0 Hz, J=2.2 Hz), 3.09-3.30 (1H, m), 3.46, 4.91 (2H, ABq, J=14.4 Hz), 3.78 (3H s), 4.09 (1H, dd, J=13 Hz, J=2.2 Hz), 4.3-4.1 (1H, m), 5.21 (2H, s), 6.85, 7.30 (4H, ABq, J=9 Hz), 7.06, 8.30 (4H, ABq, J=6 Hz) ppm.
References:
Shionogi & Co., Ltd. US4960879, 1990, A

4556-23-4
198 suppliers
$6.00/1g

838-85-7
263 suppliers
$9.00/1g

2524-64-3
386 suppliers
$14.00/5g

37968-97-1
62 suppliers
$23.00/100mg
15854-87-2
309 suppliers
$6.00/1g

37968-97-1
62 suppliers
$23.00/100mg

4556-23-4
198 suppliers
$6.00/1g
1120-87-2
171 suppliers
inquiry

37968-97-1
62 suppliers
$23.00/100mg
626-61-9
48 suppliers
$365.00/500mg

37968-97-1
62 suppliers
$23.00/100mg