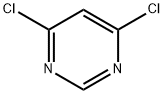
4,6-Dichloropyrimidine synthesis
- Product Name:4,6-Dichloropyrimidine
- CAS Number:1193-21-1
- Molecular formula:C4H2Cl2N2
- Molecular Weight:148.98
104-90-5
257 suppliers
$14.00/5mL
1193-24-4
534 suppliers
$6.00/25g

1193-21-1
553 suppliers
$14.00/5g
Yield:1193-21-1 100%
Reaction Conditions:
with trichlorophosphate
Steps:
2 Example 2
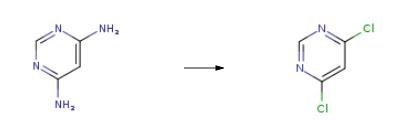
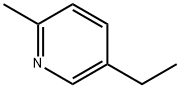
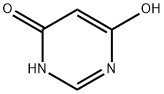
Example 2 2680 g of phosphorus oxychloride were initially introduced into the reaction vessel and 980 g of 2-methyl-5-ethyl-pyridine were added, while cooling. 460 g of 4,6-dihydroxypyrimidine were then metered in over a period of 1 hour. Further reaction and working up were carried out as described in Example 1. 4,6-Dichloropyrimidine was obtained in a yield of virtually 100% and 88% by weight of the amount of amine employed was recovered.
References:
US5719285,1998,A

1193-24-4
534 suppliers
$6.00/25g

1193-21-1
553 suppliers
$14.00/5g
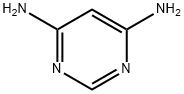
2434-56-2
207 suppliers
$25.00/1g

1193-21-1
553 suppliers
$14.00/5g
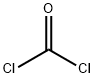
75-44-5
2 suppliers
inquiry

60100-09-6
0 suppliers
inquiry
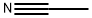
75-05-8
963 suppliers
$10.00/10g

1193-21-1
553 suppliers
$14.00/5g