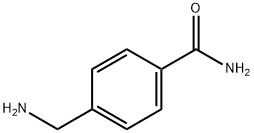
4-AMINOMETHYL-BENZAMIDE synthesis
- Product Name:4-AMINOMETHYL-BENZAMIDE
- CAS Number:369-53-9
- Molecular formula:C8H10N2O
- Molecular Weight:150.18
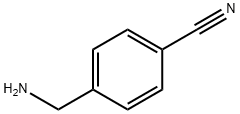
10406-25-4
162 suppliers
inquiry

369-53-9
47 suppliers
$275.00/1g
Yield: 84%
Reaction Conditions:
Stage #1:4-aminobenzyl cyanide with sulfuric acid;water at 40 - 50; for 3 h;
Stage #2: with water at 20 - 40; for 1 h;Product distribution / selectivity;
Steps:
1
In a reactor equipped with a motor-driven stirrer, 3450 g of concentrated sulfuric acid (reagent, purity: 98%) was charged and 1148 g of a hydrate of p- aminomethylbenzonitrile (958.6 g/7.253 mol as p- aminomethylbenzonitrile, purity: 83.5%, moisture: 189.4 g/10.522 mol) was gradually added so that the reaction temperature reaches 40 to 50°C, followed by mixing with stirring at 50°C for 3 hours. After the completion of the reaction, 1440 g of water was added and the solution was stirred at 40°C for one hour, then at room temperature overnight. The deposited crystal was collected by filtration and suspended in 4000 g of water and then neutralized by slowly adding 2117 g of potassium hydroxide (reagent, purity: 85%) while maintaining the temperature at 40 to 50°C. The deposited p-aminomethylbenzamide-containing potassium sulfate crystal was collected by filtration and then cooled to 5°C. The deposited crystal was collected by filtration and then dried to obtain 521.6 g of p- aminomethylbenzamide. An operation of recrystallization from the filtered p-aminomethylbenzamide-containing potassium sulfate crystal was repeated five times to obtain 421.7 g of p- aminomethylbenzamide (total yield based on p- aminomethylbenzonitrile was 84%). The purity of p-aminomethylbenzamide determined by analyzing using high-performance liquid chromatograph was 97% or higher.
References:
SHOWA DENKO K. K. WO2003/97579, 2003, A1 Location in patent:Page/Page column 12
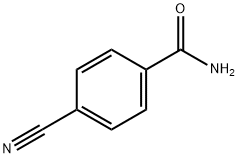
3034-34-2
106 suppliers
$55.00/250mg

369-53-9
47 suppliers
$275.00/1g
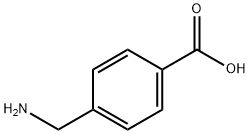
56-91-7
509 suppliers
$5.00/1g

369-53-9
47 suppliers
$275.00/1g