
4-BROMO-2-METHYL-3-NITRO-PYRIDINE synthesis
- Product Name:4-BROMO-2-METHYL-3-NITRO-PYRIDINE
- CAS Number:23056-49-7
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
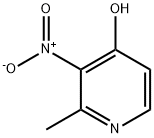
18614-66-9
29 suppliers
inquiry

23056-49-7
18 suppliers
inquiry
Yield:23056-49-7 49.7%
Reaction Conditions:
with phosphorus(V) oxybromide at 140; for 3 h;pressure vessel;
Steps:
48
[0222] A mixture of 2-methyl-3-nitropyridin-4-ol (2 g, 12.98 mmol) and phosphorus oxybromide (24.93 g, 87 mmol) was melted at 140°C in a pressure vessel over 3 hours. The reaction mixture was cooled, poured into chloroform (100 mL) and ice water (100 mL). The layers were separated and the organic layer was washed with water (2 x 25 mL), saturated sodium bicarbonate (2 x 75 mL), dried over magnesium sulfate, filtered, and concentrated to give an oil. The oil was treated with a mixture of dichloromethane and chloroform (50 mL, 3 : 1) to give a solid which was filtered and dried under vacuum. The filtrate was reduced in volume by 50% under vacuum, loaded on a silica column and eluted with 2% methanol in dichloromethane to give additional product which was concentrated and combined with the initial batch to give 4-bromo-2-methyl-3-nitropyridine (1.4 g, 6.45 mmol, 49.7 % yield); MS [M+H] found 217.0. 1H NMR (400 MHz, DMSO-d6) δ ppm 2.53 (s, 3 H) 7.88 (dd, J=5.31, 0.51 Hz, 1 H) 8.52 - 8.59 (m, 1 H).
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED;KWOK, Lily;LARSON, John David;SABAT, Mark WO2011/146287, 2011, A1 Location in patent:Page/Page column 60
18437-58-6
315 suppliers
$6.00/1g

23056-49-7
18 suppliers
inquiry

18615-86-6
144 suppliers
$21.00/250mg

23056-49-7
18 suppliers
inquiry