
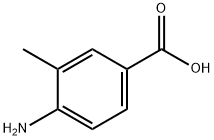
4-Carboxy-2-methylphenylisothiocyanate synthesis
- Product Name:4-Carboxy-2-methylphenylisothiocyanate
- CAS Number:1027513-23-0
- Molecular formula:C9H7NO2S
- Molecular Weight:193.22

75-15-0
234 suppliers
$40.00/100g
2486-70-6
390 suppliers
$5.00/1g

1027513-23-0
8 suppliers
$504.88/5MG
Yield:-
Reaction Conditions:
Stage #1: carbon disulfide;4-amino 3-methylbenzoic acidwith triethylamine in tetrahydrofuran;water at 20; for 28 h;
Stage #2: with iodine in tetrahydrofuran;water at 0; for 1.58333 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water;
Steps:
4
EXAMPLE 4 4-Isothiocyanato-3-methylbenzoic acid Carbon disulfide (0.26 mL, 4.4 mmol) was added to a mixture comprising 4-amino-3-methylbenzoic acid (0.23 g, 1.5 mmol), tetrahydrofuran (1.0 mL), water (1.0 mL) and triethylamine (0.51 mL, 3.6 mmol), followed by stirring at room temperature for 28 hours. The obtained reaction mixture was dropwise added to a tetrahydrofuran (1.0 mL) solution of iodine (0.41 g, 1.6 mmol) over a period of 5 minutes at 0°C, followed by stirring at 0°C for further 1.5 hours. Thereafter, 1 M hydrochloric acid (1.5 mL) and sodium sulfite (38 mg, 0.30 mmol) were added and mixed. Then, ethyl acetate (6 mL) was added, and the organic layer was separated and concentrated under reduced pressure to dryness. To the residue, ethyl acetate (6 mL) and water (1 mL) were added, and the organic layer was washed once with 1 M hydrochloric acid (1 mL) and then concentrated under reduced pressure to dryness to obtain 4-isothiocyanato-3-methylbenzoic acid as a cream-colored solid (0.30 g, yield: 103%, HPLC purity: 88%, HPLC retention time: 4.0 min). LC-MS ES- 192 (retention time: 4.3 min, condition 1).
References:
EP2248800,2010,A1 Location in patent:Page/Page column 9