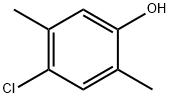
4-chloro-2,5-xylenol synthesis
- Product Name:4-chloro-2,5-xylenol
- CAS Number:1124-06-7
- Molecular formula:C8H9ClO
- Molecular Weight:156.61
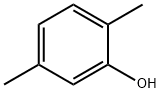
95-87-4
305 suppliers
$13.00/25g
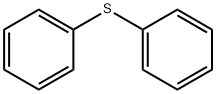
139-66-2
282 suppliers
$5.00/10g

1124-06-7
10 suppliers
$210.00/100mg
Yield:1124-06-7 121 g (94%)
Reaction Conditions:
with sulfuryl dichloride;sodium hydrogensulfite in (2S)-N-methyl-1-phenylpropan-2-amine hydrate;dichloromethane;
Steps:
1 First Step
Preparation of 4-Chloro-2,5-xylenol To a solution of 100 g (0.82 mol) of 2,5-xylenol and 0.9 g of diphenyl sulfide in 700 mL of dichloromethane was added a solution of 106 g (0.79 mol) of sulfuryl chloride in 100 mL of dichloromethane, maintaining the temperature at 5-15° C. The mixture was stirred for an additional hour and then poured onto 400 g of ice-water containing 5 g of sodium bisulfite. The layers were separated, and the organic phase was washed with water, dried (MgSO4), and concentrated to dryness. The crude solids were slurried with a minimum amount of hexanes, filtered, and suction-dried to give 121 g (94%) of product, homogeneous by Thin Layer Chromatography (TLC), GC, and 1H-NMR analysis. 1H-NMR (CDCl3) δ 2.18 (s, 3H), 2.27 (s, 3H), 4.61 (s, 1H), 6.63 (s, 1H), 7.07 (s, 1H). Lit (Blackstock, Aust. J. Chem. 1973, 26, 775): mp 74-75° C.
References:
US6489517,2002,B1

95-87-4
305 suppliers
$13.00/25g

1124-06-7
10 suppliers
$210.00/100mg

95-87-4
305 suppliers
$13.00/25g
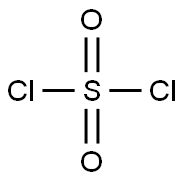
7791-25-5
252 suppliers
$15.00/0.5g
67-66-3
6 suppliers
$33.60/500ml

1124-06-7
10 suppliers
$210.00/100mg