
4-Chlorovalerophenone synthesis
- Product Name:4-Chlorovalerophenone
- CAS Number:25017-08-7
- Molecular formula:C11H13ClO
- Molecular Weight:196.67
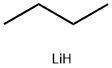
29786-93-4
0 suppliers
inquiry
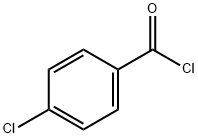
122-01-0
365 suppliers
$13.00/25g

25017-08-7
179 suppliers
$15.00/10g
Yield:25017-08-7 99 %Chromat.
Reaction Conditions:
Stage #1: 4-chloro-benzoyl chloridewith morpholine;diisobutylaluminium hydride in tetrahydrofuran;hexane at 0; for 0.166667 h;Inert atmosphere;
Stage #2: n-butyllithium in tetrahydrofuran;hexane at 0; for 0.166667 h;
Steps:
Partial alkylation of acid chlorides to corresponding ketones
General procedure: The following experimental procedure for the partial reduction of benzoyl chloride to 1-phenylpentanone is representative. A dry and argon-flushed flask, equipped with a magnetic stirring bar and a septum, was charged with morpholine (0.11 mL, 1.25 mmol) and 10 mL THF. After cooling to 0 °C, DIBALH (1.2 mL, 1.0 M in hexane, 1.2 mmol) was added dropwise and stirred for 3 h at same temperature. To the reaction mixture was slowly added benzoyl chloride (0.116 mL, 1.0 mmol) and stirred for 10 min. Then, n-BuLi (1.25 mL, 1.6 M in hexane, 2.0 mmol) was added and the mixture was stirred for 10 min again. The reaction was stopped by aqueous 1 N HCl (10 mL) and extracted with diethyl ether (2 × 10 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure. Purification of the residue by column chromatography on silica gel yielded 1-phenylpentanone (159 mg, 98%). All products in Table 3 were confirmed by comparison with NMR data reported in the literature.8
References:
Park, Jae Kyo;Shin, Won Kyu;An, Duk Keun [Tetrahedron Letters,2013,vol. 54,# 24,p. 3199 - 3203]

29786-93-4
0 suppliers
inquiry
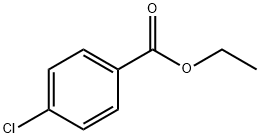
7335-27-5
132 suppliers
$10.00/1g

25017-08-7
179 suppliers
$15.00/10g
![Bis[4-fluorophenyl] sulfoxide](/CAS/GIF/395-25-5.gif)
395-25-5
27 suppliers
inquiry

25017-08-7
179 suppliers
$15.00/10g

134573-99-2
0 suppliers
inquiry

25017-08-7
179 suppliers
$15.00/10g
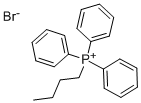
1779-51-7
401 suppliers
$7.00/10g

122334-37-6
82 suppliers
$8.00/1g

25017-08-7
179 suppliers
$15.00/10g