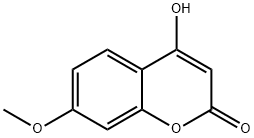
4-HYDROXY-7-METHOXYCOUMARIN synthesis
- Product Name:4-HYDROXY-7-METHOXYCOUMARIN
- CAS Number:17575-15-4
- Molecular formula:C10H8O4
- Molecular Weight:192.17
552-41-0
575 suppliers
$5.00/1g
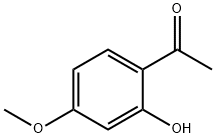
105-58-8
447 suppliers
$14.00/25g

17575-15-4
103 suppliers
$22.00/100mg
Yield: 98%
Reaction Conditions:
with sodium hydride in mineral oil at 0 - 85; for 14 h;
Steps:
4-hydroxy-7-methoxy-2H-chromen-2-one (4)
Solutionof 1-(2-hydroxy-3,4-dimethylphenyl)ethanone(5g; 0.03 mol) in diethylcarbonate (35 mL) was added dropwise to asuspension of NaH (60% dispersion in mineral oil) (5 g; 0.21 mol) indiethylcarbonate (25 mL) at 0 oC. The resulting mixture was heatedat 85 oC for 14 h after which it was cooled first to rt and then to0 oC. Water was then carefully addeddropwise to quench residual NaH. The remaining diethylcarbonate was extractedinto diethyl ether (3 x 50 mL). The aqueous phase was carefully acidified to pH3 with 2N HCl (significant foaming occurred). The resulting precipitated solidwas collected by filtration, washed sequentially with water followed bypetroleum ether (40-60 oC:3 x 50 mL), and then dried in a 90 oC oven overnight to yield whitecrystals (5.67 g; 98%). 1H-NMR(DMSO-d6) (δ/ppm): 12.38(1H, bs, OH-4), 7.72 (1H, d, H-5, J=8.4Hz), 7.00-6.91 (1H, m, H-6), 6.95 (1H, s, H-8), 5.46 (1H, s, H-3), 3.85 (1H, s,OCH3). 13C-NMR (DMSO-d6) d/ppm: 166.0 (C-4), 162.9 (C-1), 162.2 (C-7),155.4 (C-10), 124.3 (C-5), 111.8 (C-6), 108.9 (C-11), 100.5 (C-8), 88.5 (C-3),55.8 (OCH3).
References:
Andjelkovic, Uroš;Antolović, Roberto;Bracanović, Sara;Cetina, Mario;Jelić, Dubravko;Kraljević Pavelić, Sandra;Vinter, Adrijana;Wittine, Karlo;Wittine, Ozren [Bioorganic and medicinal chemistry letters,2020,vol. 30,# 18] Location in patent:supporting information
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

17575-15-4
103 suppliers
$22.00/100mg

4136-21-4
64 suppliers
$45.00/50mg

105-58-8
447 suppliers
$14.00/25g

17575-15-4
103 suppliers
$22.00/100mg

552-41-0
575 suppliers
$5.00/1g

17575-15-4
103 suppliers
$22.00/100mg

552-41-0
575 suppliers
$5.00/1g
201230-82-2
1 suppliers
inquiry

17575-15-4
103 suppliers
$22.00/100mg