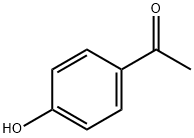
4'-Hydroxyacetophenone synthesis
- Product Name:4'-Hydroxyacetophenone
- CAS Number:99-93-4
- Molecular formula:C8H8O2
- Molecular Weight:136.15
aluminium chloride
in nitrobenzene at 20–25° or at 50–60°
in chlorobenzene between 45° and 65°, sealed tube and subjected to high power microwave irradiation for 2 min only (36%)
in nitroethane at 60° (44%)
in carbon disulfide at 45° (40%)
in petroleum ether at 50° (20%)
but between 130° and 175° (40–60%)
aluminium chloride–sodium chloride mixture at 240–250° (10%)
boron trifluoride at 90° (56%)
scandium tris(trifluoromethanesulfonate), in nitromethane, at 50° (39%)
titanium tetrachloride at 90–100° (34%)
ferric chloride at 65° (25%)
zinc chloride at 125° (8%)
hydrofluoric acid, between 20° and 100° (94%)
polyphosphoric acid, between 20° and 100° (69%) (50–53%)(44%)
Nafion-XR 500, sulfonic acid type at 100°
ZSM-5, in sulfolane, at 180° (28%)
H-ZSM-5 at 400° or at 210° (6%)
H-Nu-2 at 170° (15%)
HY (Si/Al = 3) or fluorided alumina (Al2O3-F; 3 % wt. F), at 400°.
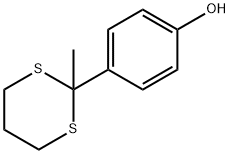
155853-28-4
0 suppliers
inquiry

99-93-4
816 suppliers
$5.00/25g
Yield:99-93-4 100%
Reaction Conditions:
with dihydrogen peroxide;tantalum pentachloride;sodium iodide in water;ethyl acetate at 20; for 1 h;
Steps:
6.2. General procedure for the deprotection of dithioacetals catalyzed by NaI-TaCl5
General procedure: A mixture of 1 (1.0 mmol), tantalum chloride (35.8 mg, 0.10 mmol), sodium iodide (15.0 mg, 0.10 mmol), and 30% hydrogen peroxide (0.46 ml, 4.0 mmol) in ethyl acetate (6 ml) and water (6 ml) was stirred at room temperature. The reaction was monitored by thin layer chromatography (TLC). After 1 disappeared on TLC, saturated aqueous sodium thiosulfate (4 ml), and saturated aqueous sodium bicarbonate (4 ml) were added to the reaction mixture. The organic layer was separated and the aqueous phase was extracted with ethyl acetate (25 ml×3). The combined organic phase was washed with brine, dried over anhydrous sodium sulfate, and evaporated. Chromatography on silica gel gave a pure product.
References:
Kirihara, Masayuki;Noguchi, Takuya;Okajima, Nobuhiro;Naito, Sayuri;Ishizuka, Yuki;Harano, Aiko;Tsukiji, Hiroyuki;Takizawa, Ryu [Tetrahedron,2012,vol. 68,# 5,p. 1515 - 1520] Location in patent:experimental part
13329-40-3
267 suppliers
$6.00/5g

99-93-4
816 suppliers
$5.00/25g
2491-38-5
259 suppliers
$5.00/100mg

99-93-4
816 suppliers
$5.00/25g
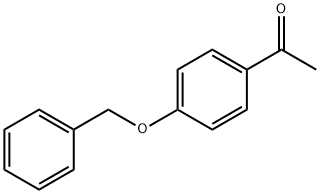
54696-05-8
201 suppliers
$10.00/1g

99-93-4
816 suppliers
$5.00/25g
![1-[4-(ALLYLOXY)PHENYL]ETHANONE](/CAS/GIF/2079-53-0.gif)
2079-53-0
15 suppliers
$45.00/100mg

99-93-4
816 suppliers
$5.00/25g