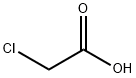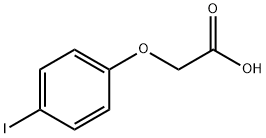
4-Iodophenoxyacetic acid synthesis
- Product Name:4-Iodophenoxyacetic acid
- CAS Number:1878-94-0
- Molecular formula:C8H7IO3
- Molecular Weight:278.04
90794-33-5
12 suppliers
$470.00/10g

1878-94-0
190 suppliers
$10.00/1g
Yield:1878-94-0 98%
Reaction Conditions:
with lithium hydroxide monohydrate in tetrahydrofuran;water at 20; for 1 h;Inert atmosphere;Schlenk technique;
Steps:
2 4.1.2 Methyl-3-[2-(4-ethynylphenoxy)acetamido]-4-hydroxybenzoate (5)
A mixture of 4 (1.53 g, 5.0 mmol), lithium hydroxide monohydrate (258 mg, 6.1 mmol) in a 1:1 solution of THF and water (12 mL) was stirred at room temperature for 5 min. The reaction was quenched by HCl solution (1 N) and the organic solvent was removed under reduced pressure. The crude products were extracted with ethyl acetate, washed with saturated aqueous NaCl solution, dried over anhydrous MgSO4, and concentrated to give product 5 as a spectroscopically pure form (1.36 g, 4.9 mmol, 98%) as a white solid: 1H NMR (400 MHz; CD3OD): δ 7.48 (d, J = 8.6 Hz, 2H), 6.66 (d, J = 8.6 Hz, 2H), 4.54 (s, 2H); 13C NMR (100 MHz; CD3OD) δ 172.3, 159.4, 139.4, 118.1, 84.1, 65.8; MS (ESI) m/z 276 [M - H]-.
References:
Nakamura, Hiroyuki;Yasui, Yuka;Ban, Hyun Seung [Journal of Organometallic Chemistry,2013,vol. 747,p. 189 - 194]
122-59-8
428 suppliers
$5.00/25g

1878-94-0
190 suppliers
$10.00/1g
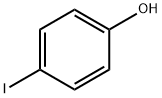
540-38-5
335 suppliers
$10.00/1g
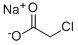
3926-62-3
258 suppliers
$19.73/250g

1878-94-0
190 suppliers
$10.00/1g

81720-18-5
14 suppliers
$45.00/50mg

1878-94-0
190 suppliers
$10.00/1g