
4-METHOXY-3-NITROBENZENE-1-CARBOTHIOAMIDE synthesis
- Product Name:4-METHOXY-3-NITROBENZENE-1-CARBOTHIOAMIDE
- CAS Number:175277-84-6
- Molecular formula:C8H8N2O3S
- Molecular Weight:212.23
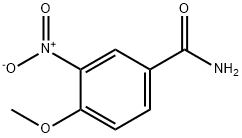
10397-58-7
45 suppliers
$51.10/1g

175277-84-6
15 suppliers
inquiry
Yield:175277-84-6 76%
Reaction Conditions:
Stage #1: 4-methoxy-3-nitro-benzoic acid amidewith Lawessons reagent in tetrahydrofuran;1,2-dimethoxyethane at 50; for 2 h;
Stage #2: with sodium hydrogencarbonate in tetrahydrofuran;1,2-dimethoxyethane at 20; for 1 h;
Steps:
Lawesson's reagent was added to a stirring mixture of 4-methoxy-3-nitrobenzamide in dimethoxyethane-THF 2:1. The reaction was stirred at 50° C. for 2 h and when completed sodium bicarbonate was added and the mixture was stirred at rt for 1 h. The solvents were removed under reduced pressure and the oily residue was slowly added to a stirring mixture of water and crushed ice (300 mL). The milky mixture was heated to boil and then left to cool to rt. resulting in yellow crystals. (0.9 g, 76% yield).
References:
US2012/316148,2012,A1 Location in patent:Page/Page column 193; 194; 195