4-TRIETHOXYSILYLANILINE synthesis
- Product Name:4-TRIETHOXYSILYLANILINE
- CAS Number:7003-80-7
- Molecular formula:C12H21NO3Si
- Molecular Weight:255.39
Yield:-
Reaction Conditions:
with tetra-(n-butyl)ammonium iodide;triethylamine;bis(acetonitrile)(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate in N,N-dimethyl-formamide at 80; for 1 h;
Steps:
2
Example 24-(diallylethoxysilyl)aniline was prepared as follows. Firstly, distilled dimethylformamide (dist. DMF: 4 mL), distilled triethylamine (dist. Et3N: 190 μL, 1.38 mmol), and triethoxysilane (169 μL, 0.92 mmol) are added dropwise to a mixture of 4-iodoaniline (100 mg, 0.46 mmol), [Rh(CH3CN)2(cod)]BF4 (5.2 mg, 0.014 mmol: cod=1,5-cyclooctadiene), tetrabutylammoniumiodide (n-BU4NI: 169 mg, 0.46 mmol). The resultant mixture was stirred under a nitrogen atmosphere at 80° C. for 1 hour to obtain a reaction mixture. Then, the solvent in the obtained reaction mixture was distilled away with a vacuum pump. A residue was extracted with ether, and then a salt thus formed was removed through a celite-filtration process. After that, an organic layer is collected, and the solvent in the organic layer is distilled away with an evaporator to obtain a crude product (I) (189 mg).Subsequently, the obtained crude product was added directly with distilled ether (dist. ether: 2 mL) under a nitrogen atmosphere, and then 1-M allylmagnasium bromide (4 mL (solvent: ether), 4 mmol) was added dropwise at 0° C. to obtain a mixture. The mixture thus obtained was stirred under a nitrogen atmosphere, at room temperature for 16 hours, and then was cooled down by using a saturated aqueous solution of sodium acid carbonate (sat. NaHCO3). After that, an organic layer was separated from the mixture while the aqueous layer was extracted with ether. The organic layer thus collected was washed with sodium acid carbonate and sodium chloride, and then the washed organic layer was dried with anhydrous magnesium sulfate (MgSO4). The dried organic layer was then filtered, and concentrated to obtain a crude product (II). Then, the obtained crude product (II) was separated and purified by PTLC (hexane/EtOAc=3/1) to obtain 4-(diallylethoxysilyl)aniline (50.2 mg, 44% yield (in two steps)).The obtained compound was subjected to 1H NMR and 13C NMR measurements. The obtained results are shown below.1H NMR (CDCl3) δ7.36 (d, J=8.1 Hz, 2H), 6.68 (d, J=8.1 Hz, 2H), 5.91-5.74 (m, 2H), 4.98-4.86 (m, 4H), 3.75 (br, 2H), 3.73 (q, J=7.0 Hz, 2H), 1.90 (d, J=7.8 Hz, 4H) 1.48 (t, J=7.0 Hz, 3H).13C NMR (CDCl3) 147.9, 135.5, 133.6, 122.9, 114.44, 114.40, 59.1, 21.4, 18.4.From the NMR measurement results, it was confirmed that the obtained compound was 4-(diallylethoxysilyl)aniline.
References:
US2008/227939,2008,A1 Location in patent:Page/Page column 13-14
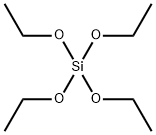
998-30-1
238 suppliers
$30.00/25g
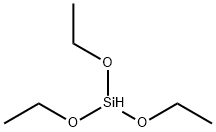
540-37-4
442 suppliers
$6.00/5g

7003-80-7
35 suppliers
inquiry

62-53-3
631 suppliers
$10.00/1g

998-30-1
238 suppliers
$30.00/25g

106-40-1
456 suppliers
$12.00/5g

7003-80-7
35 suppliers
inquiry

62-53-3
631 suppliers
$10.00/1g