
tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate synthesis
- Product Name:tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate
- CAS Number:452339-71-8
- Molecular formula:C17H23NO5
- Molecular Weight:321.37
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/NewsImg/2024-03-15/6384610503855079902291429.png)
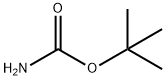
4248-19-5
374 suppliers
$5.00/5g
![2-Bromo-1-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)ethanone](/CAS2/GIF/102293-80-1.gif)
102293-80-1
84 suppliers
$265.00/250mg
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/CAS/20150408/GIF/452339-71-8.gif)
452339-71-8
62 suppliers
inquiry
Yield:452339-71-8 83%
Reaction Conditions:
Stage #1: tert-butyl carbazatewith lithium dipropan-2-ylazanide in tetrahydrofuran at -5 - 20; for 0.5 h;
Stage #2: 2-bromo-1-(2,2-dimethyl-4H-1,3-benzodioxin-6-yl)ethanone in tetrahydrofuran; for 2 h;Temperature;Reagent/catalyst;
Steps:
1-4 Example 1:
Add tert-butyl carbamate (225g, 1.1eq) to a 5L three-necked flask at room temperature,Add 500 mL of tetrahydrofuran and stir to dissolve, and bring the system to -5-0 °C,LDA (960mL, 2mol/L, 1.1eq) solution was slowly added dropwise,After adding, keep stirring for 0.5h.Then slowly add the solution prepared by 6-bromoacetyl-2,2-dimethyl-4H-benzo[1,3]dioxin (500g, 1.0eq) and 1L tetrahydrofuran into the reaction system dropwise,After the addition, the reaction was incubated for 2h. The middle control reaction is completed, the reaction solution is concentrated,Then saturated sodium bicarbonate solution (2L) and ethyl acetate (2L) were added,Stir to disperse the residue, separate the layers, and separate the organic layers with water (2L).washed with saturated brine (2L), then dried over anhydrous sodium sulfate,The magnesium sulfate was removed by filtration, and the solvent was removed by vacuum concentration.Add isopropyl ether (1L), stir at room temperature for crystallization,Filtration to obtain 467g of white powdery solid,Yield 83%, purity 99.1%.
References:
CN114736186,2022,A Location in patent:Paragraph 0012; 0018-0025

452339-70-7
9 suppliers
inquiry
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/CAS/20150408/GIF/452339-71-8.gif)
452339-71-8
62 suppliers
inquiry
![2-Bromo-1-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)ethanone](/CAS2/GIF/102293-80-1.gif)
102293-80-1
84 suppliers
$265.00/250mg
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/CAS/20150408/GIF/452339-71-8.gif)
452339-71-8
62 suppliers
inquiry

51779-32-9
272 suppliers
$5.00/1g
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/CAS/20150408/GIF/452339-71-8.gif)
452339-71-8
62 suppliers
inquiry

54030-34-1
5 suppliers
inquiry
![tert-Butyl (2-(2,2-dimethyl-4H-benzo[d][1,3]dioxin-6-yl)-2-oxoethyl)carbamate](/CAS/20150408/GIF/452339-71-8.gif)
452339-71-8
62 suppliers
inquiry