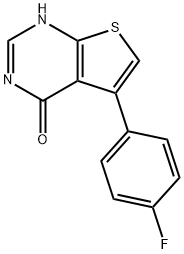
5-(4-FLUOROPHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE synthesis
- Product Name:5-(4-FLUOROPHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE
- CAS Number:35978-37-1
- Molecular formula:C12H7FN2OS
- Molecular Weight:246.26

64-18-6
1055 suppliers
$22.12/250ML
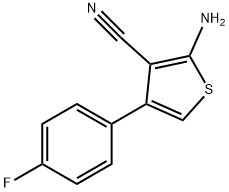
519016-84-3
1 suppliers
inquiry
![5-(4-FLUOROPHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE](/CAS/GIF/35978-37-1.gif)
35978-37-1
35 suppliers
$85.00/250mg
Yield:35978-37-1 81.8%
Reaction Conditions:
with acetic anhydride at 110; for 36 h;Inert atmosphere;
Steps:
4.1.1.3. Preparation of 5-(4-fluorophenyl)thieno[2,3-d]pyrimidin-4(3H)-one (3i)
A mixture of 2-amino-4-(4-fluorophenyl)thiophene-3-carbonitrile (210 mg, 0.96 mmol), formic acid (8 mL) and acetic anhydride (8 mL) was heated at 110 °C for 36 h. The solvent was removed under reduced pressure and the residue was purified by chromatography on silica gel to afford the title compound 3i (171 mg, 81.8%) as a white solid. 1H NMR (300 MHz, CDCl3 + CD3OD) δ: 7.02 (t, J = 9.0 Hz, 2H), 7.09 (s, 1H), 7.45 (t, J = 9.0 Hz, 2H), 7.86 (s, 1H).
References:
Zhao, Ailing;Gao, Xin;Wang, Yuanxiang;Ai, Jing;Wang, Ying;Chen, Yi;Geng, Meiyu;Zhang, Ao [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 13,p. 3906 - 3918] Location in patent:experimental part

60100-09-6
0 suppliers
inquiry
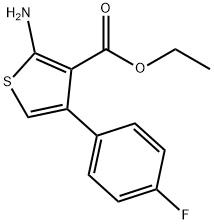
35978-33-7
70 suppliers
$28.60/50MG
![5-(4-FLUOROPHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE](/CAS/GIF/35978-37-1.gif)
35978-37-1
35 suppliers
$85.00/250mg
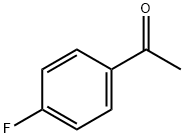
403-42-9
534 suppliers
$5.00/10g
![5-(4-FLUOROPHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE](/CAS/GIF/35978-37-1.gif)
35978-37-1
35 suppliers
$85.00/250mg
![Propanedinitrile, 2-[1-(4-fluorophenyl)ethylidene]-](/CAS/20200331/GIF/579-48-6.gif)
579-48-6
1 suppliers
inquiry
![5-(4-FLUOROPHENYL)-3H-THIENO[2,3-D]PYRIMIDIN-4-ONE](/CAS/GIF/35978-37-1.gif)
35978-37-1
35 suppliers
$85.00/250mg