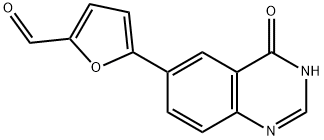
5-(4-Hydroxy-6-quinazolinyl)furan-2-carbaldehyde synthesis
- Product Name:5-(4-Hydroxy-6-quinazolinyl)furan-2-carbaldehyde
- CAS Number:1334953-71-7
- Molecular formula:C13H8N2O3
- Molecular Weight:240.21
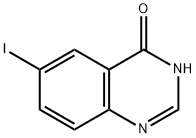
16064-08-7
224 suppliers
$10.00/1g
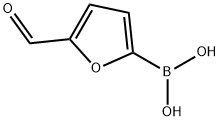
27329-70-0
458 suppliers
$6.00/1g

1334953-71-7
5 suppliers
inquiry
Yield:1334953-71-7 97.6%
Reaction Conditions:
Stage #1: 5-formylfurane-2-boronic acidwith palladium diacetate;tri tert-butylphosphoniumtetrafluoroborate in water;dimethyl sulfoxide at 20; for 0.333333 h;Inert atmosphere;
Stage #2: 6-iodoquinazolin-4(3H)-onewith potassium acetate in water;dimethyl sulfoxide at 75 - 85; for 0.333333 h;Inert atmosphere;
Steps:
1 Synthesis of 5-(4-oxo-3,4-dihydroquinazolin-6-yl)furan-2-carbaldehyde
At ambient temperature,The 5: 2 v / v mixture solvent (1400 mL) of dimethylsulfoxide (DMSO) was mixed with H2O for 30 minutes using nitrogen.Under nitrogen protection,5-formylfuran-2-ylboronic acid ((VIa);26.8 g, 193 mmol) to a mixed solvent,Dissolve.Add [HP (t-Bu) 3] BF4 (840 mg, 2.94 mmol)And palladium acetate Pd (OAc) 2 (680 mg, 2.94 mmol)And at ambient temperature,After stirring the mixture for 20 minutes,Potassium acetate (AcOK, 18.8 g, 192 mmol) was added to the reactor,Stirring was continued for 20 minutes,6-iodoquinazolin-4 (3H) -one ((Va))40 g, 147 mmol).The reaction mixture was heated to 80 ± 5 ° C (internal temperature).After the HPLC monitoring reaction was complete,The reaction mixture was filtered while hot,The hot water (400 mL, 80 + 5 [deg.] C) was then added to the filtrate.It was slowly cooled to 0-15 ° C (solid began to precipitate at an internal temperature of 70 ° C)Then filtered,The filter cake was washed successively with H2O (80 mL), MeCN (60 mL).The filter cake was vacuum dried at 60 ± 5 ° C for 6 hours,5- (4-oxo-3,4-dihydroquinazolin-6-yl) -furan-2-carbaldehyde ((IX); 34.6 g, 144 mmol)HPLC analysis purity of 99.7%The yield was 97.6%.
References:
CN104418845,2017,B Location in patent:Paragraph 0033-0035