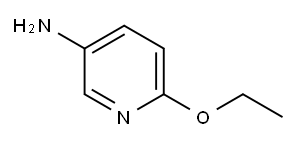
5-Amino-2-ethoxypyridine synthesis
- Product Name:5-Amino-2-ethoxypyridine
- CAS Number:52025-34-0
- Molecular formula:C7H10N2O
- Molecular Weight:138.17

31594-45-3
164 suppliers
$16.00/1g

52025-34-0
91 suppliers
$60.00/1 g
Yield: 100%
Reaction Conditions:
with methanol;5%-palladium/activated carbonHeating / reflux;
Steps:
32 EXAMPLE 32;2-Cyano-3-(6-ethoxy-pyridin-3-ylamino)-acrylic acid ethyl ester
Hydrogenation of 2-ethoxy-5-nitropyridine (6.2 g, 36.9 mmol) with 10 %Pd/C (500 mg) in abs MeOH (350 ml) at atmospheric pressure gave 2-ethoxy-5-aminopyridine in an quantitative yield. The product was pure (TLC, NMR), therefore was used for the next step without further purification. 2-Ethoxy-5-aminopyridine was heated at reflux with ethyl(ethoxymethylene)cyanoacetate (12 g, 2 mole eq.) in toluene for 10 hrs. The reaction mixture was cooled, and the resulting solid was collected, which was pure on TLC and H-NMR. mass spectrum (electrospray, m/e): 262.0 (M+H); IR cm-1: 2989, 2213, 1672, 1628, 1610; H-NMR δ(CDCl13): 1.235 (3H, t, CH3), 1.304 (3H, t, CH3), 4.166 (2H, q, CH2), 4.274 (2H, q, CH2), 6.815 (1H, d, C3-H), 7.760 and 7.880 (1H, d,d, cis and trans olefin H), 8.228 (1H, s, C2-H), 8.276 (1H, dd, C4-H), 10.50 (1H, bs, NH).
References:
American Cyanamid Company US6355636, 2002, B1 Location in patent:Page column 59
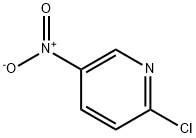
4548-45-2
531 suppliers
$6.00/5g

52025-34-0
91 suppliers
$60.00/1 g
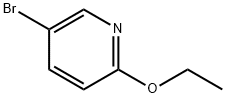
55849-30-4
271 suppliers
$12.72/1gm:

52025-34-0
91 suppliers
$60.00/1 g