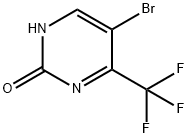
5-BroMo-4-(trifluoroMethyl)pyriMidin-2(1H)-one synthesis
- Product Name:5-BroMo-4-(trifluoroMethyl)pyriMidin-2(1H)-one
- CAS Number:785777-90-4
- Molecular formula:C5H2BrF3N2O
- Molecular Weight:242.98
104048-92-2
221 suppliers
$10.00/5g

785777-90-4
39 suppliers
inquiry
Yield:-
Reaction Conditions:
with bromine;potassium acetate;acetic acid at 80; for 2 h;
Steps:
6
The title compound was prepared using a modified procedure based on Ondi, L. et al., Eur. J. Org. Chem. 2 A mixture of 4-(trifluoromethyl)pyrimidin-2-ol (6.05g, 36.9mmol), KOAc (10.85g, 3eq.), acetic acid (80mL), and bromine (5.9g, leq.) was heated for 2h at 80 °C. After being cooled to rt, the mixture was concentrated. The residue was partitioned between EtOAc and water. The aqueous layer was extracted with EtOAc (2x). The combined organic layers were washed with brine, dried over Na2S04. After filtration and concentration, the crude white solid product (9.38g, quant, yield) was used for next steps without further purification. A mixture of 5-bromo-4-(trifluoromethyl)pyrimidin-2-ol (1.35g, 5.56mmol), POCI3 (15mL), and DMF(2 drops, cat. Amount) was heated for 2h at 80 °C. The mixture was cooled to 0 °C by ice/water bath. Some ice pellets were added to the stirred mixture (exotherm). After stirring for 20min. (the ice added should have melted), some sat. aq. NaHCC"3 (c.a. 15mL) was added carefully to neutralize some acid. The mixture was extracted with hexanes (3x). The combined organic layers were washed with brine, dried over Na2S04. After filtration and concentration (by rotavapor only The product is volatile), 5-bromo-2- chloro-4-(trifluoromethyl)pyrimidine, as a clear oil (1.32g, 91% yield) was used as crude for next steps without further purification.
References:
WO2013/138568,2013,A1 Location in patent:Page/Page column 66; 67