
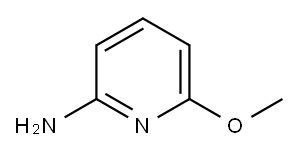
5-BROMO-6-METHOXYPYRIDIN-2-AMINE synthesis
- Product Name:5-BROMO-6-METHOXYPYRIDIN-2-AMINE
- CAS Number:1211533-83-3
- Molecular formula:C6H7BrN2O
- Molecular Weight:203.04

67-56-1
734 suppliers
$9.00/25ml
89284-11-7
101 suppliers
$17.00/100mg

1211533-83-3
79 suppliers
$42.00/250mg
Yield:1211533-83-3 82%
Reaction Conditions:
Stage #1: methanolwith sodium at -20 - 0; for 4 h;
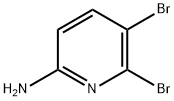
Stage #2: 5,6-dibromopyridin-2-ylamine in methanol at 120; for 24 h;Sealed tube;
Stage #3: with water in methanol;
Steps:
7b
Example 7b 5-Bromo-6-methoxy-pyridin-2-ylamine Sodium metal (1.8 g, 78.2 mmol) was added to dry MeOH (70 mL) at -20° C. in small portions. The solution was stirred for 4 h at 0° C. and added to a pressure vessel containing 5,6-dibromo-pyridin-2-ylamine (Example 7a, 10 g, 39.8 mmol). The reaction mixture was heated in a sealed tube at 120° C. for 24 h. The solvent was removed under reduced pressure and the residue was diluted with water. The aqueous phase was neutralized using 2 M HCl and extracted with EtOAc (3*100 mL). The combined extracts were dried over MgSO4, filtered and concentrated in vacuo. The residue was purified by flash column chromatography using a gradient of 5 to 15% acetone in hexane to afford the title compound (6.6 g, 82%). 1H NMR (400 MHz, CDCl3) δ ppm 3.91 (s, 3H), 4.31 (br. s., 2 H), 5.99 (d, 1H), 7.48 (d, 1H). ESMS m/z 202.8, 204.8 [M+H]+.
References:
US2012/122843,2012,A1 Location in patent:Page/Page column 15
17920-35-3
214 suppliers
$9.00/1g

1211533-83-3
79 suppliers
$42.00/250mg

89284-11-7
101 suppliers
$17.00/100mg

124-41-4
681 suppliers
$12.00/25g

1211533-83-3
79 suppliers
$42.00/250mg

67-56-1
734 suppliers
$9.00/25ml

89284-11-7
101 suppliers
$17.00/100mg

124-41-4
681 suppliers
$12.00/25g

1211533-83-3
79 suppliers
$42.00/250mg
19798-81-3
431 suppliers
$5.00/1g

1211533-83-3
79 suppliers
$42.00/250mg