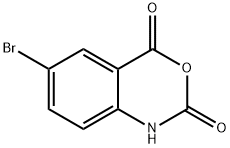
5-Bromoisatoic anhydride synthesis
- Product Name:5-Bromoisatoic anhydride
- CAS Number:4692-98-2
- Molecular formula:C8H4BrNO3
- Molecular Weight:242.03
1HNMR(DMSO-d6): ! 11.85 (s, br., 1H), 7.96 (dd, J = 0.8, 8.7 Hz, 1H), 7.87 (dd, J = 2.3, 8.7 Hz, 1H), 7.09 (dd, J = 8.8, 0.8 Hz, 1H).
32315-10-9
413 suppliers
$10.00/1g
5794-88-7
460 suppliers
$6.00/10g

4692-98-2
213 suppliers
$13.00/1g
Yield:4692-98-2 98%
Reaction Conditions:
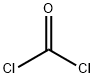
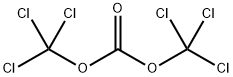
with pyridine in dichloromethane;acetonitrile at 50 - 55; for 2 h;
Steps:
2.a
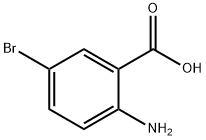
a) Preparation of 6-bromo-1H-benzo[d][1,3]oxazine-2,4-dione A solution of 2-amino-5-bromo-benzoic acid (280 g, 1.3 mol) in acetonitrile (1.3 l) was warmed up to 50-55° C. Pyridine (206 g, 2.61 mol) and a solution of triphosgene (128.8 g, 0.43 mol) in dichloromethane (720 ml) were simultaneously added dropwise. After completion of the addition, the mixture was stirred at 50-55° C. for an additional 2 h. The solvent was removed under reduced pressure and water was added. The precipitate was collected by filtration, successively washed with water and chilled dichloromethane, and then dried under vacuum to afford the desired product 6-bromo-1H-benzo[d][1,3]oxazine-2,4-dione (304 g, 98%). This material was used in the next step without further purification.
References:
Chen, Li;Chen, Shaoqing;Michoud, Christophe US2006/63804, 2006, A1 Location in patent:Page/Page column 11

87-48-9
280 suppliers
$10.00/5g

4692-98-2
213 suppliers
$13.00/1g
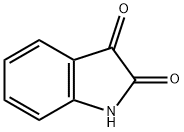
91-56-5
432 suppliers
$5.00/25g

4692-98-2
213 suppliers
$13.00/1g
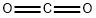
124-38-9
122 suppliers
$175.00/23402
201230-82-2
1 suppliers
inquiry
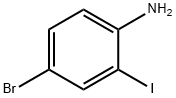
66416-72-6
184 suppliers
$6.00/250mg

4692-98-2
213 suppliers
$13.00/1g