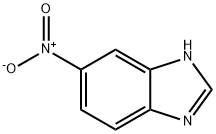
5-Nitrobenzimidazole synthesis
- Product Name:5-Nitrobenzimidazole
- CAS Number:94-52-0
- Molecular formula:C7H5N3O2
- Molecular Weight:163.13
Yield:94-52-0 90%
Reaction Conditions:
with mesoporous nanocomposite of Fe3O4 and -NHSO3H functionalized silica (MCM-41) in neat (no solvent) at 90; for 0.333333 h;
Steps:
General procedure for the synthesis of benzimidazole, benzoxazole, andbenzothiazole derivatives 2(a-o)
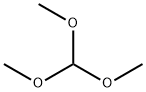
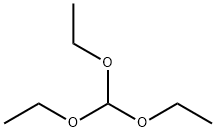
General procedure: To a mixture of 1,2-phenylenediamine, 2-aminophenol, or 2-aminothiophenol, asappropriate (1 mmol), and the desired ortho ester (trimethyl orthoformate, triethylorthoformate, or triethyl orthoacetate) (1.5 mmol), in neat conditions, Fe3O4MCM-41NH-SO3H (0.03 g) was added and the resulting mixture was stirred in an oil bath at90 C for the proper time as determined by TLC [EtOAc: n-hexane (1:2)]. After endingthe reaction, ethyl acetate (20 mL) was added and the catalyst was separated by an externalmagnet. The organic phase was washed with brine and dried over Na2SO4. After filtration,the solvent was removed from the product under reduced pressure to obtainthe pure product. In some cases, the product was purified by recrystallization from nhexane.The known compounds were identified by matching their IR and NMR datawith literature values from the references cited, as well as melting points for those compoundswhich were solids. For the sake of completeness, the spectral data of some representativecompounds follow.
References:
Pourhasan-Kisomi, Reyhaneh;Shirini, Farhad;Golshekan, Mostafa [Organic Preparations and Procedures International,2021,vol. 53,# 2,p. 166 - 175]
51-17-2
565 suppliers
$14.00/25g

94-52-0
239 suppliers
$8.00/5g
99-56-9
391 suppliers
$6.00/5g

94-52-0
239 suppliers
$8.00/5g