
6-Chloro-1-tetralone synthesis
- Product Name:6-Chloro-1-tetralone
- CAS Number:26673-31-4
- Molecular formula:C10H9ClO
- Molecular Weight:180.63
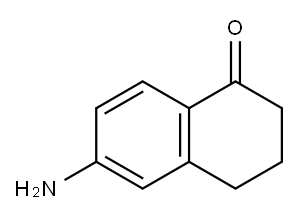
3470-53-9
214 suppliers
$6.00/250mg

26673-31-4
204 suppliers
$8.00/100mg
Yield:26673-31-4 88%
Reaction Conditions:
with tert.-butylnitrite;copper dichloride in acetonitrile at 60; for 0.75 h;
Steps:
13.a Step a4
CuCl2 (2.0 g, 15.0 mmol) and t-butyl nitrite (2.2 mL, 15.0 mmol) in dry CH3CN were heated at 60° C., followed by the addition of 6-aminotetralone (2.0 g, 12.0 mmol). The reaction mixture was heated for 45 min, then cooled down at room temperature, quenched with 2N HCl (200 mL) and Et2O (200 mL). The aqueous layer was extracted with Et2O and the combined organic layer was washed with water, brine, dried over anhydrous Na2SO4, concentrated by rotary evaporation, and purified by column chromatography to give 6-chloro-3,4-dihydronaphthalen-1(2H)-one (16) (88%). 111 NMR (600 MHz, CDCl3) δ 7.96 (d, J=6.0 Hz, 1H), 7.28-7.26 (m, 2H), 2.94 (t, J=6.0 Hz, 2H), 2.65 (t, J=6.0 Hz, 2H), 2.16-2.11 (m, 2H), 7.12-7.07 (m, 2H), 6.92 (d, J=12.0 Hz, 1H), 6.85 (s, 1H), 5.67 (s, 1H). GC-MS (ES) for C10H9C10 [M]+ 180.
References:
US2015/105351,2015,A1 Location in patent:Paragraph 0440

29480-09-9
4 suppliers
inquiry

26673-31-4
204 suppliers
$8.00/100mg

22991-05-5
28 suppliers
inquiry

26673-31-4
204 suppliers
$8.00/100mg
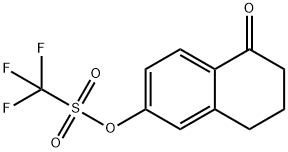
144464-64-2
9 suppliers
inquiry

26673-31-4
204 suppliers
$8.00/100mg

1355061-67-4
0 suppliers
inquiry

26673-31-4
204 suppliers
$8.00/100mg