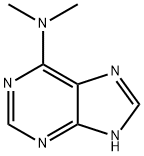
6-Dimethylaminopurine synthesis
- Product Name:6-Dimethylaminopurine
- CAS Number:938-55-6
- Molecular formula:C7H9N5
- Molecular Weight:163.18
68-94-0
641 suppliers
$5.00/100mg

938-55-6
222 suppliers
$50.00/250mg
Yield:-
Steps:
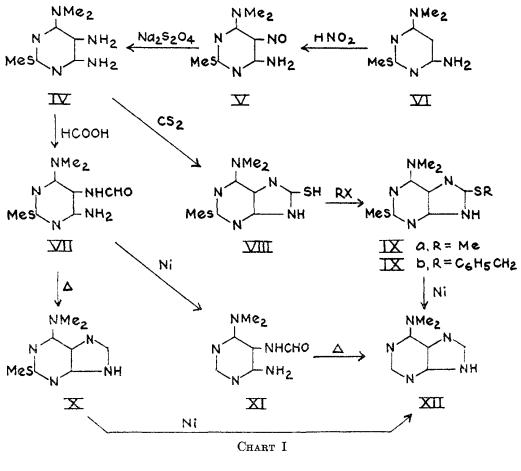
Multi-step reaction with 2 steps
1: 47 percent / POCl3, dimethylaniline / 0.5 h / Heating
2: 81 percent / ethanol / 12 h / 150 °C / high pressure
References:
LaMontagne, Maurice P.;Smith, David C.;Wu, Geng-Shuen [Journal of Heterocyclic Chemistry,1983,vol. 20,p. 295 - 299]

124-40-3
501 suppliers
$18.00/100ml
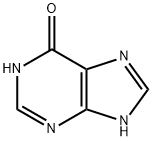
87-42-3
464 suppliers
$14.00/25g

938-55-6
222 suppliers
$50.00/250mg
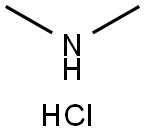
506-59-2
526 suppliers
$5.00/5G

68-94-0
641 suppliers
$5.00/100mg

938-55-6
222 suppliers
$50.00/250mg

506-59-2
526 suppliers
$5.00/5G

87-42-3
464 suppliers
$14.00/25g

938-55-6
222 suppliers
$50.00/250mg

81693-59-6
0 suppliers
inquiry

124-40-3
501 suppliers
$18.00/100ml
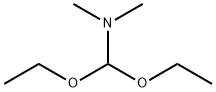
1188-33-6
247 suppliers
$11.19/5ML

938-55-6
222 suppliers
$50.00/250mg