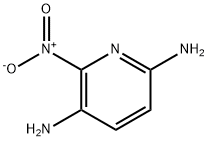
6-Nitro-2,5-diaminopyridine synthesis
- Product Name:6-Nitro-2,5-diaminopyridine
- CAS Number:69825-83-8
- Molecular formula:C5H6N4O2
- Molecular Weight:154.13
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

69825-83-8
46 suppliers
inquiry
Yield:-
Reaction Conditions:
with sodium hydroxide;immobilized glutaryl amidase in water; pH=7.5 at 37; for 24 h;Product distribution / selectivity;Enzymatic reaction;potassium phosphate buffer;
Steps:
B.230
A solution of 5-[5-amino-6-nitro-2-(pyridinyl)amino]-5-oxopentanoic acid (according to example 1) was prepared at a concentration of 100 mM in aqueous 200 mM potassium phosphate buffer, and the pH was adjusted to 7.5 by the addition of 6 M sodium hydroxide solution. The pH-adjusted glutaramide solution was diluted 1:10 (final concentration of 10 mM) into a 200 mM potassium phosphate buffer solution, pH 7.5, containing a suspension of immobilized glutaryl acylase (BioCatalytics, Inc., Pasadena, CA USA; Product Number 1464213) added to achieve a catalytic activity of 104 units per milliliter. As a negative control, an identical reaction mixture was prepared without the immobilized glutaryl acylase. The reaction mixtures were mixed gently at 37 DEG C and the progress of the reaction was followed by thin layer chromatography (silica gel plates, eluted with ethylacetate; visualization by UV-light). Complete conversion of the 5-[5-amino-6-nitro-2-(pyridinyl)amino]-5-oxopentanoic acid to 2,5-diamino-6-nitropyridine was achieved within 24 hours. In the case of the control reaction without enzyme, no conversion of the mono-glutaramide of 2,5-diamino-6-nitropyridine was detected over a 24 hour period.
References:
Wella Aktiengesellschaft EP1348700, 2003, A2 Location in patent:Page/Page column 22
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

69825-83-8
46 suppliers
inquiry
![[1,2,5]OXADIAZOLO[3,4-B]PYRIDINE, 3-OXIDE](/CAS/GIF/27808-53-3.gif)
27808-53-3
2 suppliers
inquiry

69825-83-8
46 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

69825-83-8
46 suppliers
inquiry