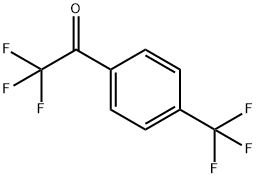
4-(TRIFLUOROMETHYL)-ALPHA,ALPHA,ALPHA-TRIFLUOROACETOPHENONE synthesis
- Product Name:4-(TRIFLUOROMETHYL)-ALPHA,ALPHA,ALPHA-TRIFLUOROACETOPHENONE
- CAS Number:74853-66-0
- Molecular formula:C9H4F6O
- Molecular Weight:242.12
![2,2,2-trifluoro-1-[4-(trifluoromethyl)phenyl]ethan-1-ol](/CAS/20200331/GIF/150698-81-0.gif)
150698-81-0
15 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

74853-66-0
71 suppliers
$23.00/100mg
Yield: 44% , 40 %Spectr.
Reaction Conditions:
with oxone;potassium 2-iodo-5-methylbenzenesulfonate in acetonitrile at 90; for 18 h;
Steps:
3.4. Typical procedure for the hypervalent iodine(V)-catalyzed oxidation
General procedure: Method A: a mixture of 2,2,2-trifluoro-1-(4-methoxyphenyl)ethanol (2c) (0.206 g, 1.0 mmol) and sodium 2-iodobenzenesulfonate (8) (as monohydrate, 0.016 g, 0.05 mmol, 5 mol%), powdered Oxone (0.554 g, 0.9 mmol) in CH3CN (5 mL) was stirred at 90 °C under the atmosphere of air. After stirring for 18 h, the reaction mixture was allowed to cool to room temperature. The reaction mixture was filtered, being successively washed with ethyl acetate. The combined filtrates were washed with water (3× 10 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by silica gel column chromatography (eluent: hexane/EtOAc = 10/1) to give 2,2,2-trifluoro-1-(4-methoxyphenyl)-1-ethanone (1c) (0.165 g, 0.81 mmol, 81% yield).
References:
Tanaka, Yusuke;Ishihara, Takashi;Konno, Tsutomu [Journal of Fluorine Chemistry,2012,vol. 137,p. 99 - 104] Location in patent:experimental part
![2,2,2-trifluoro-1-[4-(trifluoromethyl)phenyl]ethan-1-ol](/CAS/20200331/GIF/150698-81-0.gif)
150698-81-0
15 suppliers
inquiry

74853-66-0
71 suppliers
$23.00/100mg
![Benzene, 1-[2,2-difluoro-1-[(trimethylsilyl)oxy]ethenyl]-4-(trifluoromethyl)-](/CAS/20210305/GIF/243845-55-8.gif)
243845-55-8
0 suppliers
inquiry

74853-66-0
71 suppliers
$23.00/100mg
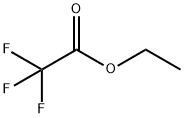
383-63-1
472 suppliers
$10.00/25 g
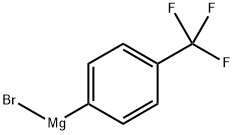
402-51-7
24 suppliers
inquiry

74853-66-0
71 suppliers
$23.00/100mg
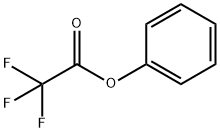
500-73-2
98 suppliers
$10.00/1g
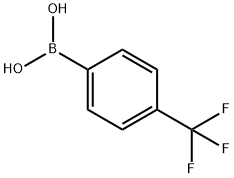
128796-39-4
420 suppliers
$10.00/5g

74853-66-0
71 suppliers
$23.00/100mg