
4-(6-Acryloyloxyhexyloxy)-benzoesure (4-cyanophenylester) synthesis
- Product Name:4-(6-Acryloyloxyhexyloxy)-benzoesure (4-cyanophenylester)
- CAS Number:83847-14-7
- Molecular formula:C23H23NO5
- Molecular Weight:393.43

The target monomer was prepared by firstly converting the acid into the corresponding acid chloride, then condensing this withp-cyanophenol. The acid (17.32 g, 0.059 mol) was stirred with thionyl chloride (35.7 g, 0.3 mol) in the presence of DMF (2 drops) and 2,6-di-tert-butyl-4-methyl phenol (4.4 g, 0.02 mol). After ca. 25 min, by which time solution was achieved, the excess thionyl chloride was removed by rotary evaporation and high vacuum (1-2 h). The acid chloride residue was dis- solved in anhydrous chloroform (30 mL), then added slowly dropwise into a stirred solution of p-cyanophenol (7.03 g, 0.059 mol) and triethylamine (6.07 g, 0.06 mol) in chloroform (20 mL) at 0°C. After the addition the mixture was stirred at room temperature for 24 h and then chloroform (200 mL) added. This solution was washed with water (50 mL) followed by 2 N sodium hydroxide solution (3 x 50 mL) and water (2 x 50 mL). The organic layer was dried (MgSO,), evaporated, then subjected to flash chromatography, using dichloro- methane as both the solvent and the eluant. The monomer was finally recrystallized from isopropanol and shown to be pure by tlc. Yield: 16 g, 69%.

2009-83-8
359 suppliers
$7.00/25g

83847-14-7
57 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 3 steps
1: 54 percent / potassium iodide, potassium hydroxide / ethanol; H2O / 20 h / Heating
2: 67 percent / hydroquinone, p-toluenesulphonic acid / benzene / 20 h / Heating
3: 1.) thionyl chloride, 2,6-di-tert-butyl-4-methyl phenol, DMF; 2.) triethylamine / 1.) 25 min; 2.) chloroform, room t., 24 h
References:
Cowie, J. M. G.;Hunter, H. W. [Canadian Journal of Chemistry,1995,vol. 73,p. 1811 - 1817]
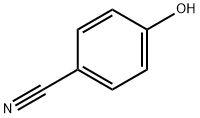
767-00-0
554 suppliers
$6.00/25g
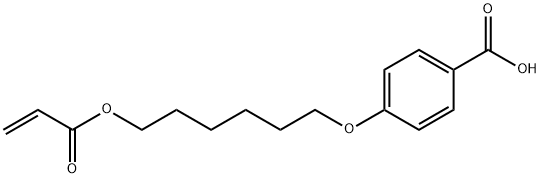
83883-26-5
139 suppliers
$8.00/250mg

83847-14-7
57 suppliers
inquiry
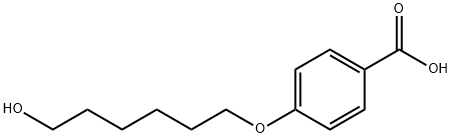
83883-25-4
45 suppliers
$79.00/1G

83847-14-7
57 suppliers
inquiry
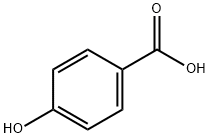
99-96-7
764 suppliers
$5.00/10g

83847-14-7
57 suppliers
inquiry