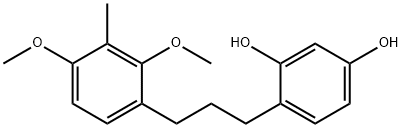
4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol synthesis
- Product Name:4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol
- CAS Number:869743-37-3
- Molecular formula:C18H22O4
- Molecular Weight:302.36
1400999-35-0
2 suppliers
inquiry
![4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol](/CAS/20180703/GIF/869743-37-3.gif)
869743-37-3
24 suppliers
inquiry
Yield: 93%
Reaction Conditions:
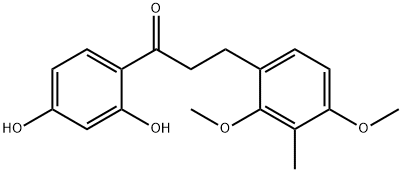
Stage #1:1-(2,4-dihydroxyphenyl)-3-(2,4-dimethoxy-3-methylphenyl)propan-1-one with sodium bis(2-methoxyethoxy)aluminium dihydride in tetrahydrofuran at 0; for 18.5 h;Inert atmosphere;Reflux;Industry scale;
Stage #2: with hydrogenchloride;water in tetrahydrofuran at 0 - 5; for 0.5 h;Industry scale;
Steps:
4
Example 4 Synthesis of 4-(3-(2,4-dimethoxy-3-methylphenyl)propyl)benzene-1,3-diol Under a nitrogen atmosphere, (IB-7) (1 equiv., 25 kg) was added into a reaction vessel containing THF (250 L) while stirring. The reaction mixture was then cooled to 0-5° C. and charged with Vitride (i.e., sodium bis(2-methoxyethoxy)aluminum hydride, 1 equiv., 120 L) over 2.5 h. The reaction mixture was then heated and maintained at reflux until found complete by HPLC (16 h). Next, the reaction was cooled to 0-5° C., quenched with 150 L of concentrated HCl (30-36%) in water (150 L) and stirred for 30 min. The reaction mixture was then diluted with water (150 L) and extracted twice with ethyl acetate (450 L and 375 L). The combined ethyl acetate extract was washed with aqueous sodium bicarbonate (25 kg in 150 L of water) followed by saturated sodium chloride (150 L). Charcoal (3.25 Kg) was added to the organic layer and the mixture was refluxed for 20 minutes. This mixture was filtered through Celite and washed with ethyl acetate (20 L). The ethyl acetate layer was then dried with anhydrous sodium sulfate (25 kg) and concentrated under vacuum at 40-45° C. The crude product thus obtained was triturated for 4 h with toluene (5 L) at room temperature and filtered. This residue was washed with toluene (16 L), and the solid thus obtained was dried under vacuum for 8 h at 55-60° C. to produce 16.9 Kg of crude product.The crude product (16.9 Kg) from the previous stage was dissolved in acetone (4 L) with heating to 55° C. This solution was then cooled to room temperature (24° C.) and treated with water (60 L) while stirring. Stirring was continued for 10 h at room temperature before the resulting solids were allowed to settle to the bottom, and the top organic layer was carefully removed. The remaining mixture was then diluted with petroleum ether (13 L), stirred for an additional 5 h at room temperature, filtered and then dried under vacuum for 12 h at 60° C. Product from two such crystallizations was combined, dried, ground and sieved to obtain purified (II-1) (15.64 Kg, 93%). MP: 108.0° C.; 1H-NMR (CDCl3, 500 MHz): δ 6.96 (d, 1H, J=8.3 Hz), 6.85 (d, 1H, J=8.3 Hz), 6.62 (d, 1H, J=8.3 Hz), 6.27 (d, 1H, J=2.2 Hz), 6.21 (dd, 1H, J=2.2 Hz), 3.77 (s, 3H), 3.63 (s, 3H), 2.52-2.58 (m, 4H), 2.10 (s, 3H), 1.78-1.82 (m, 2H); 13C-NMR (CDCl3, 125 MHz): δ 158.55 (C), 158.43 (C), 157.38 (C), 157.15 (C), 131.53 (CH), 128.95 (C), 128.28 (CH), 121.45 (C), 120.30 (C), 107.40 (CH), 103.60 (CH), 61.44 (CH3), 56.19 (CH3), 32.90 (CH2), 30.77 (CH2), 30.51 (CH2), 9.195 (CH3).
References:
Unigen, Inc. US2012/245393, 2012, A1 Location in patent:Page/Page column 15-16
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol](/CAS/20180703/GIF/869743-37-3.gif)
869743-37-3
24 suppliers
inquiry
89-84-9
568 suppliers
$6.00/10g
![4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol](/CAS/20180703/GIF/869743-37-3.gif)
869743-37-3
24 suppliers
inquiry

22877-01-6
79 suppliers
$21.00/250mg
![4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol](/CAS/20180703/GIF/869743-37-3.gif)
869743-37-3
24 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![4-[3-(2,4-Dimethoxy-3-methylphenyl)propyl]-1,3-benzenediol](/CAS/20180703/GIF/869743-37-3.gif)
869743-37-3
24 suppliers
inquiry