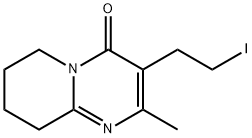
885706-66-1 synthesis
- Product Name:885706-66-1
- CAS Number:885706-66-1
- Molecular formula:C11H15IN2O
- Molecular Weight:318.15
66698-27-9
14 suppliers
inquiry

885706-66-1
1 suppliers
inquiry
Yield:885706-66-1 88%
Reaction Conditions:
with iodine;triphenylphosphine in acetonitrile at 0; for 16 h;
Steps:
1.3
3rd step: production of 3-(2-iodoethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[l,2- a]pyrimidine-4-one; 15 g (0.0594 mol) powdered iodine is added during stirring to the suspension of 15.5 g (0.0594 mol) triphenylphosphine in 100 ml of anhydrous acetonitrile and cooled to 0 °C.The suspension is stirred at 0 °C for 90 minutes and then the product obtained in the 2nd step is added to this suspension. The cooling is shutting off and then the suspension is stirred for 16 hours and then the solvent is distilled off under reduced pressure (30 mbar, 40 0C water-bath). 100 ml of ethyl acetate and 60 ml of water are added to the residue and after stirring and decantation, water layer is separated, the organic layer is extracted with 4x40 ml of water and 40 ml of IN HCl. The collected water layers are extracted with 20 ml of ethyl acetate. 60 ml of dichloromethane is added to the water layer and the EPO
References:
WO2006/46082,2006,A1 Location in patent:Page/Page column 9-10