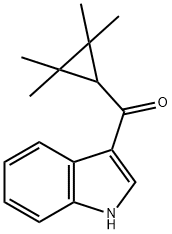
(1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone synthesis
- Product Name:(1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone
- CAS Number:895152-66-6
- Molecular formula:C16H19NO
- Molecular Weight:241.33
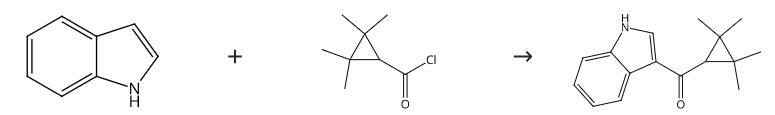
120-72-9
614 suppliers
$6.00/25g

24303-61-5
43 suppliers
$31.80/1gm:

895152-66-6
89 suppliers
$65.00/1mg
Yield:895152-66-6 85%
Reaction Conditions:
Stage #1: indolewith zirconium(IV) chloride in dichloromethane at 0; for 0.0833333 h;Inert atmosphere;
Stage #2: 2,2,3,3-tetramethylcyclopropanecarbonyl chloride in dichloromethane at 0; for 4 h;Inert atmosphere;
Steps:
(1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone (6c)
To a stirred solution of indole (600 mg, 5.12 mmol) in DCM (8 mL) at 0 °C under nitrogen,ZrCl4 (1789.7 mg, 7.68 mmol) was added. After 5 minutes, 2,2,3,3-tetramethylcyclopropanecarbonyl chloride (1644.9 mg, 10.24 mmol) in DCM (3 mL) wasadded dropwise. The temperature was kept at 0 °C until completion of reaction (4 h) indicatedby TLC. The reaction mixture was quenched with H2O (10 mL) and extracted with EtOAc (3x 15 mL). The combined organic layers were washed with H2O (5 x 10 mL) and concentratedunder reduced pressure. The crude product was purified with flash column chromatography(EtOAc:n-heptane, 2:3) and 6c (1050.6 mg, 85%) was isolated.
References:
Dahlén, Johan;Konradsson, Peter;Liu, Huiling;Rexander, Anders;Vestling, Erik;Wallgren, Jakob;Wu, Xiongyu [Synlett,2020,vol. 31,# 5,p. 517 - 520] Location in patent:supporting information
15641-58-4
163 suppliers
$16.00/5g

895152-66-6
89 suppliers
$65.00/1mg