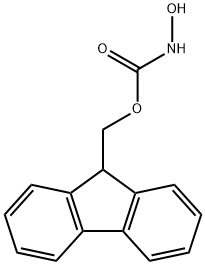
9-FLUORENYLMETHYL N-HYDROXYCARBAMATE synthesis
- Product Name:9-FLUORENYLMETHYL N-HYDROXYCARBAMATE
- CAS Number:190656-01-0
- Molecular formula:C15H13NO3
- Molecular Weight:255.27
82911-69-1
648 suppliers
$7.00/25g

190656-01-0
61 suppliers
$8.00/250mg
Yield:190656-01-0 80%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydrogencarbonate in water;ethyl acetate at 5 - 20; for 4 h;
Steps:
Solid phase synthesis of HCA-Phe-NHOH and HCA-Pro-NHOH
Hydroxylamine hydrochloride (834 mg, 12 mmol) was dissolved in 40 mL of aqueous sodium hydrogen carbonate (2.2 g, 26 mmol), and cooled to 5 °C. N-(9-fluorenylmethoxycarbonyloxy) succinimide (Fmoc-OSu, 4.0 g, 12 mmol) dissolved in 40 mL ethyl acetate was added drop wise to the rapidly stirred hydroxylamine solution in an ice-bath and stirred for 4 h at room temperature. The reaction was monitored by TLC (ethyl acetate/hexane = 1:1, Rf = 0.4). After the water layer was removed, the organic layer was washed with saturated aqueous potassium hydrogen sulfate and brine. This organic extract was concentrated in high vacuum, and then N-Fmoc protected hydroxylamine (Fmoc-NHOH) was obtained as a white crystalline solid after trituration in hexane and stored overnight (80% yield). Its structure was identified by 1H NMR (JNM-LA300 spectrometer, JEOL Ltd, Tokyo, Japan): (δH, CDCl3) 4.21 (1H, t, Fmoc CH), 4.32 (2H, d, Fmoc CH2), 7.28-7.43, 7.68, 7.86 (8H, m, Fmoc Ar. CH), 8.77 (1H, s, NH), 9.75 (1H, br s, OH). Fmoc-NHOH (2 equiv) was coupled to 2-chlorotrityl chloride (CTC) resin (1.43 mmol/g) with N,N’-diisopropylethylamine (DIPEA; 4 equiv) in dichloromethane (DCM) for 48 h. Fmoc-NHOH loaded CTC resin was treated with 10% DIPEA/methanol (v/v) to block the remaining chloride groups. The resulting resin was filtered, and its loading level was 1.0 mmol/g, which was determined by Fmoc titration. After treating with 20% piperidine/N-methyl-2-pyrrolidone (NMP) for 30 min to remove Fmoc groups, Fmoc-l-Pro-OH or Fmoc-l-Phe-OH (2 equiv) was coupled to the resin with 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3,-tetramethyl uronium hexafluorophosphate methanaminium (HATU), 1-hydroxy-7-azabenzotriazole (HOAt), and DIPEA (4 equiv) for 1.5 h at room temperature. After removing Fmoc groups by 20% piperidine/NMP, HCA (2 equiv) was coupled to the amino acid anchored resin with benzotriazole-1-yl-oxy-tris-(dimethylamino)-phosphonium hexafluorophosphate (BOP; 2 equiv), hydroxybenzotriazole (HOBt; 2 equiv) and DIPEA (3 equiv) for 5 h. The final product was cleaved from the resin by 30% trifluoroacetic acid (TFA)/DCM (v/v) for 1 h. The resin was filtered, and the filtrate was concentrated in high vacuum, followed by precipitation with cold diethyl ether. The resulting HCA-Phe-NHOH and HCA-Pro-NHOH were identified by QUATTRO Triple Quardrupole Tandem mass spectrometer (Micromass & Waters, Milford, MA, USA) at National Instrumentation Center for Environmental Management (NICEM): CA-Phe-NHOH (m/z calcd: 343.1 [M+H]+; found: 343.0), CA-Pro-NHOH (m/z calcd: 293.1 [M+H]+; found: 293.1), DHCA-Phe-NHOH (m/z calcd: 345.1 [M+H]+; found: 345.1), DHCA-Pro-NHOH (m/z calcd: 295.1 [M+H]+; found: 295.1), pCoA-Phe-NHOH (m/z calcd: 327.1 [M+H]+; found: 327.1), pCoA-Pro-NHOH (m/z calcd: 277.1 [M+H]+; found: 277.0), FA-Phe-NHOH (m/z calcd: 357.1 [M+H]+; found: 357.1), FA-Pro-NHOH (m/z calcd: 307.1 [M+H]+; found: 307.0), SA-Phe-NHOH (m/z calcd: 387.1 [M+H]+; found: 387.0), SA-Pro-NHOH (m/z calcd: 337.1 [M+H]+; found: 337.1). Their purities were analyzed by RP-HPLC (Thermo Scientific Spectra System AS300; Thermo-Fisher, Waltham, MA, USA) using C18 reverse phase column (120 Å, 5 μm, 4.6 × 250 mm; AAPPTec, Louisville, KY, USA) using the following conditions: gradient elution with A: 0.1% TFA/water, B: 0.1% TFA/acetonitrile; from 10% to 90% over 30 min, a flow rate: 1.0 mL/min; detection: UV, 280 or 326 nm. HCA-Phe-NHOH were purified by a semi-preparative RP-HPLC column using an A to B gradient (A: 0.1% TFA in water, B: 0.1% TFA in acetonitrile; from 10% to 90% B over 30 min, at a flow rate of 4.0 mL/min) and freeze-dried.
References:
Kwak, Seon-Yeong;Yang, Jin-Kyoung;Choi, Hye-Ryung;Park, Kyung-Chan;Kim, Young-Bu;Lee, Yoon-Sik [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 4,p. 1136 - 1142]
28920-43-6
550 suppliers
$5.00/5g

190656-01-0
61 suppliers
$8.00/250mg