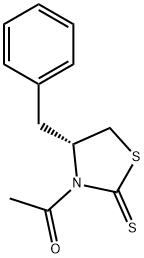
1-[(4R)-4-(phenylmethyl)-2-thioxo-3-thiazolidinyl]-Ethanone synthesis
- Product Name:1-[(4R)-4-(phenylmethyl)-2-thioxo-3-thiazolidinyl]-Ethanone
- CAS Number:911040-42-1
- Molecular formula:C12H13NOS2
- Molecular Weight:251.37
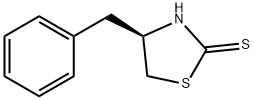
110199-17-2
118 suppliers
$30.00/250mg

75-36-5
559 suppliers
$17.92/100G
![1-[(4R)-4-(phenylmethyl)-2-thioxo-3-thiazolidinyl]-Ethanone](/CAS/20180703/GIF/911040-42-1.gif)
911040-42-1
19 suppliers
inquiry
Yield:911040-42-1 95%
Reaction Conditions:
Stage #1:(R)-4-benzyl-thiazolidine-2-thione with n-butyllithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2:acetyl chloride in tetrahydrofuran at 0; for 1 h;
Steps:
4.1. (R)-1-(4-Benzyl-2-thioxo-thiazolidin-3-yl)ethanone 7
To a stirred solution of (R)-4-benzyl-thiazolidine-2-thione 10 (2.75 g, 13.2 mmol) in dry THF (30 mL) at 0 °C under a nitrogen atmosphere was added slowly n-BuLi (6.3 mL, 15.7 mmol, 2.5 M) and stirred for 30 min at the same temperature. Next, acetyl chloride (1.24 g, 15.7 mmol) was added slowly at 0 °C and stirred for 1 h. After completion of the reaction, the reaction mixture was quenched with a saturated aq solution NH4Cl (15 mL). The organic layer was separated and the aqueous layer was extracted with EtOAc (3 × 25 mL). The combined organic layers were washed with water (2 × 30 mL), brine (2 × 30 mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (100-200 mesh, eluent: 5% EtOAc in hexane) to afford (R)-1-(4-benzyl-2-thioxo-thiazolidin-3-yl)-ethanone 7 (3.13 g, 95%) as bright yellow crystals. (c 1.0, CHCl3); IR (KBR): νmax 2925, 1701, 1362, 1215, 1018, 701 cm-1; 1H NMR (300 MHz, CDCl3): δ 7.51-7.03 (m, 5H, Ar-H), 5.37-5.29 (m, 1H), 3.36 (ddd, J = 11.3, 7.5, 0.9 Hz, 1H), 3.21 (dd, J = 13.2, 3.7 Hz, 1H), 3.02 (dd, J = 10.3, 0.9 Hz, 1H), 2.87 (d, J = 11.3 Hz, 1H), 2.78 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 201.51, 170.66, 136.45, 129.39, 128.85, 127.16, 68.15, 36.62, 31.76, 27.02; MASS (LCMS): m/z 274 (M+Na)+.
References:
Venkatesham, Akkaladevi;Srinivasa Rao, Ramisetti;Nagaiah, Kommu [Tetrahedron Asymmetry,2012,vol. 23,# 5,p. 381 - 387] Location in patent:experimental part
5267-64-1
426 suppliers
$10.40/1G
![1-[(4R)-4-(phenylmethyl)-2-thioxo-3-thiazolidinyl]-Ethanone](/CAS/20180703/GIF/911040-42-1.gif)
911040-42-1
19 suppliers
inquiry
673-06-3
589 suppliers
$14.00/25G
![1-[(4R)-4-(phenylmethyl)-2-thioxo-3-thiazolidinyl]-Ethanone](/CAS/20180703/GIF/911040-42-1.gif)
911040-42-1
19 suppliers
inquiry