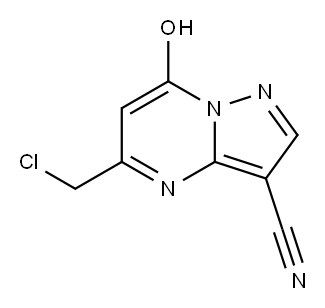
5-(chloroMethyl)-7-hydroxypyrazolo[1,5-a]pyriMidine-3-carbonitrile synthesis
- Product Name:5-(chloroMethyl)-7-hydroxypyrazolo[1,5-a]pyriMidine-3-carbonitrile
- CAS Number:925675-85-0
- Molecular formula:C8H5ClN4O
- Molecular Weight:208.6
Yield:925675-85-0 95%
Reaction Conditions:
in acetic acid at 80; for 4 h;Reflux;
Steps:
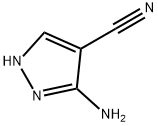
3-cyano-5-chloromethyl-7-hydroxypyrazolo[1,5-a]pyrimidine
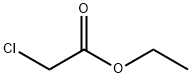
In a 250 ml three-necked flask, 100 ml of glacial acetic acid was used as a solvent, and 3-amino-4-cyano-pyrazole (10.8 g,0.1 mol) and ethyl chloroacetate (23 g, 0.15 mol) were added and heated to about 80 °C. The raw material was substantially dissolved,and a solid was formed in arefluxing reaction for 4hours. After the reaction was completed by TLC, the reaction mixture was cooled to room temperature, filtered, rinsed with glacial acetic acid, and dried to give a milky yellow solid (19.7 g, 95% yield).
References:
CN108484613,2018,A Location in patent:Paragraph 0047; 0048; 0049