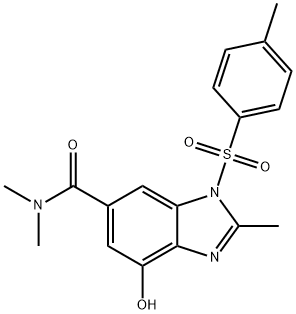
7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide synthesis
- Product Name:7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide
- CAS Number:942195-86-0
- Molecular formula:C18H19N3O4S
- Molecular Weight:373.43
![4-(benzyloxy)-N,N,2-trimethyl-1-tosyl-1H-benzo[d]imidazole-6-carboxamide](/CAS/20180601/GIF/942195-85-9.gif)
942195-85-9
6 suppliers
inquiry
![7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide](/CAS/GIF/942195-86-0.gif)
942195-86-0
51 suppliers
inquiry
Yield:942195-86-0 98%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in tetrahydrofuran at 20; under 760.051 Torr; for 30 h;
Steps:
8.4
A mixture of 4-(benzyloxy)-λ/,λ/,2-trimethyl-1 -[(4-methylphenyl)sulfonyl]-1 H- benzimidazole-6- carboxamide (29.0 g, 62.6 mmol, Step 3) and 10% palladium on carbon (6.0 g) in tetrahydrofuran (200 mL)15 was stirred under hydrogen gas (1 atm) at room temperature for 24 hours. Another 4.0 g of 10% palladium on carbon was added, and the mixture was stirred under hydrogen gas (1 atm) at room temperature for additional 6 hours. The resulted mixture was filtered through a pad of Celite, and the filtrate was concentrated in vacuo to afford the title compound as a white solid (23.0 g, 98 %). 1H NMR (CDCI3, 270 MHz) δ: 7.82 (d, J = 8.1 Hz, 2H), 7.63 (s, 1 H), 7.31 (d, J = 8.1 Hz, 2H), 6.92 (s, 1 H),20 3.14 (br. s, 3H), 3.01 (br. s, 3H), 2.79 (s, 3H), 2.40 (s, 3H) ppm (-OH was not observed). MS (ESI) m/z: 374 (M+H)+, 372 (M-H)".
References:
WO2008/35195,2008,A1 Location in patent:Page/Page column 45
713530-47-3
19 suppliers
inquiry
![7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide](/CAS/GIF/942195-86-0.gif)
942195-86-0
51 suppliers
inquiry
774582-60-4
18 suppliers
inquiry
![7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide](/CAS/GIF/942195-86-0.gif)
942195-86-0
51 suppliers
inquiry
942195-80-4
7 suppliers
inquiry
![7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide](/CAS/GIF/942195-86-0.gif)
942195-86-0
51 suppliers
inquiry
![4-(benzyloxy)-6-bromo-2-methyl-1-tosyl-1H-benzo[d]imidazole](/CAS/20180531/GIF/942195-88-2.gif)
942195-88-2
4 suppliers
inquiry
![7-hydroxy-N,N,2-triMethyl-3-tosyl-3H-benzo[d]iMidazole-5-carboxaMide](/CAS/GIF/942195-86-0.gif)
942195-86-0
51 suppliers
inquiry