
Acetaldehyde synthesis
- Product Name:Acetaldehyde
- CAS Number:75-07-0
- Molecular formula:C2H4O
- Molecular Weight:44.05

74-96-4
431 suppliers
$9.00/5g

75-07-0
411 suppliers
$14.00/5mL
Yield:75-07-0 93%
Reaction Conditions:
with C30H38Cl2Ir2N4 in dimethyl sulfoxide at 30; for 4.5 h;
Steps:
2 Example 2: Dinuclear and half-filled ruthenium complexes containing bisimine ligands catalyze the oxidation of halogenated hydrocarbons to aldehydes
The catalyst prepared in Example 1 was used to catalyze the oxidation of a halogenated hydrocarbon:To a solution of dinuclear and half-filled ruthenium complex (0.001 mmol, 0.9 mg) in DMSO, was added to ethyl bromide (1 mmol, 109 mg).Open reaction, reaction temperature 30 ° C, reaction time is 270 minutes,After the reaction, the reaction solution is washed with water, extracted and concentrated.The crude product is separated by silica gel column chromatography and dried to a constant mass.The corresponding aldehyde compound C2H4O (41 mg, yield 93%) was obtained.
References:
CN109575087,2019,A Location in patent:Paragraph 0027; 0028; 0029
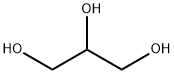
56-81-5
1624 suppliers
$5.00/25g

75-07-0
411 suppliers
$14.00/5mL

64-19-7
1527 suppliers
$10.00/25ML
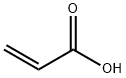
79-10-7
683 suppliers
$20.50/500ml
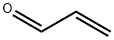
107-02-8
4 suppliers
$38.60/4s8501

56-81-5
1624 suppliers
$5.00/25g

75-07-0
411 suppliers
$14.00/5mL

79-10-7
683 suppliers
$20.50/500ml
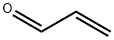
107-02-8
4 suppliers
$38.60/4s8501

64-17-5
698 suppliers
$10.00/50g

75-07-0
411 suppliers
$14.00/5mL

56-81-5
1624 suppliers
$5.00/25g

75-07-0
411 suppliers
$14.00/5mL
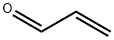
107-02-8
4 suppliers
$38.60/4s8501