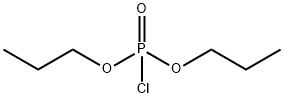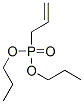
Allylphosphonic acid dipropyl ester synthesis
- Product Name:Allylphosphonic acid dipropyl ester
- CAS Number:1473-63-8
- Molecular formula:
- Molecular Weight:0
Yield:1473-63-8 94.2%
Reaction Conditions:
with 1-butyl-3-methylimidazolium carbonate;triethylamine in cyclohexane at 20; for 2 h;Inert atmosphere;
Steps:
5 Example 5
Under the protection of nitrogen, add 100mmol of allyl alcohol, 10mmol of 1-butyl-3-methyl-imidazolium carbonate, 30ml of cyclohexane and 150mmol of triethylamine into the reaction vessel and stir evenly. At room temperature, it will dissolve 30ml of cyclohexane di-n-propyl chlorophosphate (100mmol) solution was added dropwise to the reaction vessel, and reacted for 2h. After the reaction, the system was filtered to remove solid triethylamine hydrochloride, and triethylamine hydrochloride The salt was washed with cyclohexane 3 times, the washing liquid was collected, saturated sodium bicarbonate solution was added to the filtrate, stirred for 10 min, and then left to stand for liquid separation, the organic phase was collected, the aqueous phase was extracted 3 times with cyclohexane, and the extract was collected. The washing liquid, the organic phase and the extract are combined, dried over anhydrous magnesium sulfate, decolorized by activated carbon, and distilled under reduced pressure to obtain the product di-n-propyl allylphosphonate. The calculated yield is 94.2% and the purity is 99.4%. .
References:
CN112759611,2021,A Location in patent:Paragraph 0050-0051