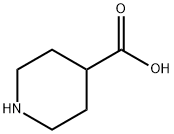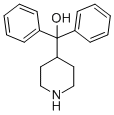
alpha,alpha-Diphenyl-4-piperidinomethanol synthesis
- Product Name:alpha,alpha-Diphenyl-4-piperidinomethanol
- CAS Number:115-46-8
- Molecular formula:C18H21NO
- Molecular Weight:267.37
Yield:115-46-8 415 g
Reaction Conditions:
Stage #1:bromobenzene with magnesium in tetrahydrofuranReflux;
Stage #2:isonipecotic acid in tetrahydrofuran;toluene at 50 - 120; under 304.02 - 1140.08 Torr; for 11 h;Inert atmosphere;
Steps:
Synthesis of diphenyl(piperidin-4-yl)methanol (4c):
Ina 3 L round bottom flask equipped with mechanical stirrer and a water bath the following materials are poured: magnesium turnings (100 g, 4.16 mmol), tetrahydrofuran (650 mL), bromobenzene (43.6 g, 0.277 mmol). The mixture is heated to reflux while stirring and as soon as the reaction is started a solution of brombenzene (608.4 g, 3.87 mmol) is slowly added at such a rate so as to maintain reflux. When the addition is complete,the mixture is refluxed until magnesium is almost completely dissolved. Then isonipecotic acid (4a) (200 g) and toluene(300 mL) are added to phenyl magnesium bromide (4b) (Grignardre agent) kept at 50 °C. The mixture is refluxed at atmospheric pressure for about 1 h. Then, the reaction is kept under pressure with nitrogen (0.4 to 1.5 atm) and the mixture is heated again between 105 and 120 °C. for 10 h. After this, the reaction mixtureis allowed to cool, the pressure of the reaction is slowly decreased and the mixture is slowly poured into 4 L of cold water. Yield 415 g of the azacyclonol [16] compound 4c of the title (Scheme-I).
References:
Sri Ramudu;Ramachandran;Venkat Rao;Murali Krishna;Satya Narayana;Reddy, Kallam Naveen [Asian Journal of Chemistry,2017,vol. 29,# 10,p. 2113 - 2115]

96067-93-5
6 suppliers
inquiry

115-46-8
400 suppliers
$10.00/1g

112818-77-6
2 suppliers
inquiry

115-46-8
400 suppliers
$10.00/1g
24228-40-8
259 suppliers
$9.59/250mg

115-46-8
400 suppliers
$10.00/1g
1126-09-6
490 suppliers
$15.40/25g

115-46-8
400 suppliers
$10.00/1g