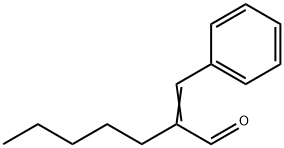
alpha-Amylcinnamaldehyde synthesis
- Product Name:alpha-Amylcinnamaldehyde
- CAS Number:122-40-7
- Molecular formula:C14H18O
- Molecular Weight:202.29
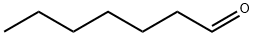
111-71-7
214 suppliers
$13.50/100ML

100-52-7
945 suppliers
$17.30/2g

122-40-7
345 suppliers
$20.00/25mL
Yield:122-40-7 97%
Reaction Conditions:
with benzoic acid;L-proline in neat (no solvent) at 125; for 1 h;Inert atmosphere;Aldol Condensation;Reagent/catalyst;Time;
Steps:
The aldol condensation was carried out by taking freshly distilled benzaldehyde (15.8 mmol) along with 40 mol% of the amino acid as a catalyst, 40 mol% of benzoic acid as co-catalyst and n-decane as an internal standard in a three-necked round bottom flask. The reaction was carried out under inert atmosphere at a desired temperature to minimize the oxidation of aldehydes used in the reaction. In order to improve the selectivity for the cross-aldol product and maximize atom efficiency of the reaction, 1-alkanaldehydes (7.9 mmol) were added to the reaction mixture under a controlled condition (1130 μl/h) using a syringe pump. The reaction mixture was colled down to room temperature after completion of the reaction (checked by GC). All the reactions were done in triplicate to ensure the reproducibility of the reaction.
References:
Ganga, Venkata Subba Rao;Abdi, Sayed H.R.;Kureshy, Rukhsana I.;Khan, Noor-Ul H.;Bajaj, Hari C. [Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical,2016,vol. 55A,# 8,p. 950 - 955]
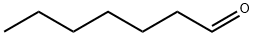
111-71-7
214 suppliers
$13.50/100ML

100-52-7
945 suppliers
$17.30/2g

3021-89-4
4 suppliers
inquiry

122-40-7
345 suppliers
$20.00/25mL
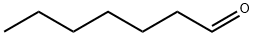
111-71-7
214 suppliers
$13.50/100ML

100-52-7
945 suppliers
$17.30/2g
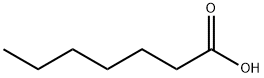
111-14-8
390 suppliers
$14.00/25G

122-40-7
345 suppliers
$20.00/25mL
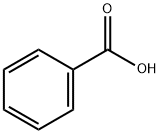
65-85-0
1326 suppliers
$10.00/25 g
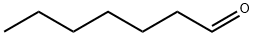
111-71-7
214 suppliers
$13.50/100ML

100-52-7
945 suppliers
$17.30/2g

111-14-8
390 suppliers
$14.00/25G

122-40-7
345 suppliers
$20.00/25mL
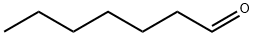
111-71-7
214 suppliers
$13.50/100ML

100-52-7
945 suppliers
$17.30/2g

3021-89-4
4 suppliers
inquiry

111-14-8
390 suppliers
$14.00/25G

122-40-7
345 suppliers
$20.00/25mL