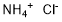
Ammonium chloride synthesis
- Product Name:Ammonium chloride
- CAS Number:12125-02-9
- Molecular formula:ClH4N
- Molecular Weight:53.49
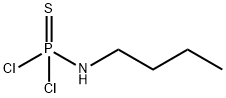
6141-81-7
0 suppliers
inquiry
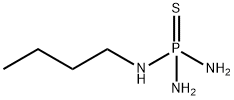
94317-64-3
288 suppliers
$6.00/5g
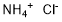
12125-02-9
1243 suppliers
$10.00/250G
Yield:94317-64-3 70%
Reaction Conditions:
with ammonia in toluene at -40 - 70;
Steps:
6.b
Into a 4-litre sulfonation flask equipped with a mechanical stirrer, a thermometer, a dosing funnel with a calcium chloride stopper and cooled to the temperature of -40 0C, 300 ml of dry toluene (water content < 500 ppm) and 15 equivalents of liquid ammonia (30 mol) were poured. 2250 ml of cooled (-17 °C) NBPCl2 solution obtained in the previous step was added at cooling and stirring by such a rate that the temperature of the reaction mixture does not rise over -18 °C. After all the NBPCl2 toluene solution was added, cooling of the reaction mixture was ceased and the resulting suspension was stirred for 1 hour without any tempering. During another 1 hour, the suspension was warmed to the room temperature, and during one more hour, the suspension was heated to 65-70 °C. A fume hood is recommended. After 30 minutes, the suspension was heat-filtered. The filtration cake of NH4Cl was washed with 50 ml of hot toluene (70 0C). The combined toluene filtrates were cooled to 3-5 °C, the resulting product suspension was stirred for 3 hours and then filtered. The filtration cake of the product was washed with 50 ml of cold toluene (3- 50C), thoroughly sucked, dried, weighed and analysed. 234.0 g of white crystalline product NBPT (70 % of the calculated yield) was obtained, with the purity of 97.0 % (HPLC). A purity of NBPT greater than 99.0 % was achieved by simple re- crystallization from toluene.
References:
WO2010/45895,2010,A2 Location in patent:Page/Page column 11

7727-37-9
111 suppliers
$323.00/56l
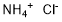
12125-02-9
1243 suppliers
$10.00/250G
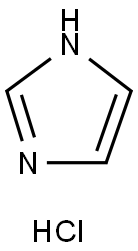
1467-16-9
158 suppliers
$6.00/10g
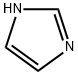
288-32-4
969 suppliers
$5.00/25g
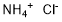
12125-02-9
1243 suppliers
$10.00/250G
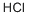
7647-01-0
11 suppliers
$10.00/10g

7727-37-9
111 suppliers
$323.00/56l
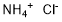
12125-02-9
1243 suppliers
$10.00/250G
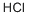
7647-01-0
11 suppliers
$10.00/10g
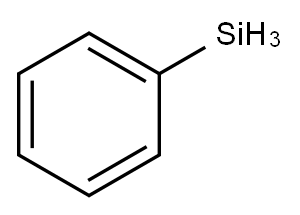
694-53-1
227 suppliers
$17.46/1G
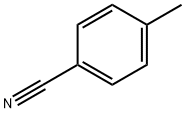
104-85-8
313 suppliers
$11.00/25g
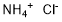
12125-02-9
1243 suppliers
$10.00/250G