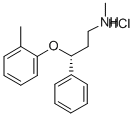
Atomoxetine hydrochloride synthesis
- Product Name:Atomoxetine hydrochloride
- CAS Number:82248-59-7
- Molecular formula:C17H22ClNO
- Molecular Weight:291.82
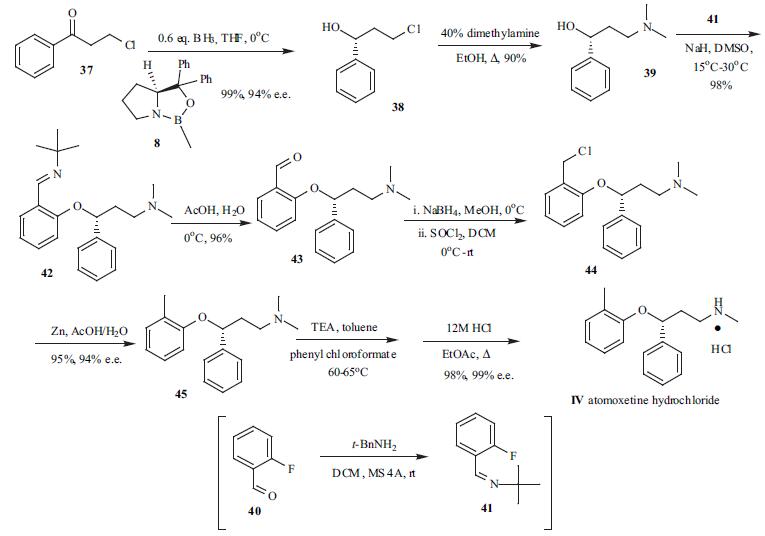
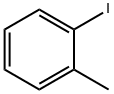
615-37-2
291 suppliers
$5.00/5g
115290-81-8
72 suppliers
$130.00/2.5mg

82248-59-7
457 suppliers
$14.00/25mg
Yield:82248-59-7 99%
Reaction Conditions:
Stage #1:ortho-methylphenyl iodide;(R)-N-methyl-3-phenyl-3-hydroxypropylamine with potassium carbonate;copper(l) iodide in toluene at 148; for 21 h;Heating / reflux;Ullmann type reaction;
Stage #2: with hydrogenchloride in water; pH=1 - 2
Stage #3: with sodium hydroxide in water; pH=11 - 12
Steps:
5
(R)-N-methyl-3-(2-methylphenoxy)-benzenepropanamine hydrochloride:; A 3 -necked 100 ml glass reactor was flushed for 15 min with N2 and subsequently charged with 15 g (90.8 mmol) of the above mentioned (3R)-methyl-3-hydroxy-3- phenylpropylamine (>99 % ee, chiral HPLC), potassium phosphate (28.9 g, 136.2 mmol) and 1.73 g copper(I)iodide (9.8 mmol, 10 mol-%). 60 ml of toluene was added to the mixture and the suspension was stirred for 5 min. 12.8 ml (100 mmol) of 2-iodotoluene was added and the reaction mixture was heated to reflux for 24 h. After cooling to room temperature, the suspension was filtered and the filter cake was washed with 60 ml of toluene. 75 ml of water was added to the filtrate and the mixture was stirred for 10 min at room temperature. The aqueous phase was brought to pH 1-2 with 30 % HCl and the phases were separated. 60 ml of toluene was added to the aqueous phase and aqueous NaOH was added until pH 12-14 of the aqueous phase was reached. After stirring for EPO
References:
FERMION OY WO2007/10082, 2007, A1 Location in patent:Page/Page column 18-19

872996-05-9
0 suppliers
inquiry

82248-59-7
457 suppliers
$14.00/25mg

83113-55-7
0 suppliers
inquiry

82248-59-7
457 suppliers
$14.00/25mg
![Carbamic acid, methyl[3-(2-methylphenoxy)-3-phenylpropyl]-, 1,1-dimethylethyl ester, (R)- (9CI)](/CAS/20210305/GIF/134619-78-6.gif)
134619-78-6
0 suppliers
inquiry

82248-59-7
457 suppliers
$14.00/25mg
95-48-7
434 suppliers
$17.00/25g

74-89-5
7 suppliers
$13.44/25ML

51699-49-1
1 suppliers
inquiry

82857-39-4
61 suppliers
inquiry

82248-59-7
457 suppliers
$14.00/25mg