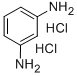
Benzene-1,3-diamine dihydrochloride synthesis
- Product Name:Benzene-1,3-diamine dihydrochloride
- CAS Number:541-69-5
- Molecular formula:C6H10Cl2N2
- Molecular Weight:181.06
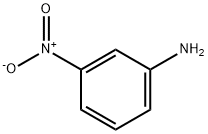
99-09-2
10 suppliers
$14.00/1g

541-69-5
108 suppliers
$34.00/25g
Yield:541-69-5 90%
Reaction Conditions:
Stage #1: 3-nitro-anilinewith hydrazine in water at 20 - 80; for 2 h;Green chemistry;
Stage #2: with hydrogenchlorideGreen chemistry;Temperature;Solvent;
Steps:
Reduction of 3-nitroaniline 1 in the presence of the nickel nanoparticles and PVP/Ni composite.
To a suspension of 0.1 g (0.7 mmol) of 3-nitroaniline 1 in 0.5 mL of H2O, 1.0 mL of the hydrous sol of the particles (0.07 mmol, 0.1 equiv.) was added at room temperature, and after 5 min 0.3 mL (5-fold excess) of hydrazine hydrate was poured into the resulting mass. After 15 min, the reaction mixture was foamed; it was stirred at 70-80°C for 4 h (TLC control), and upon completion of the reaction the catalyst was separated by filtration, and the precipitate was washed with ethanol. The filtrate was evaporated to reduce by 2/3 its original volume and acidified with 0.4 mL of concentrated HCl to pH 1, and the resulting 1,3-phe-nylenediamine dihydrochloride precipitate was washed with dilute HCl (1 : 1), dried, and recrystallized from a methanol-hexane mixture.1,3-Phenylenediamine dihydrochloride (11). Yield 88-90%, white crystals, mp >205°C (decomp.). IR spectrum, ν, cm -1 : 3410, 2965 b (NH), 1608, 1584, 1600, 1490, 1200, 1185. Mass spectrum, m/z (I rel , %): 108.05 (90) [M - H] + . Found, %: C 39.79; H 5.59; N 15.46; Cl 39.17. C6H10Cl2N2. Calculated, %: C 39.80; H 5.57; N 15.47; Cl 39.16.
References:
Ignatovich, Zh. V.;Ermolinskaya;Katok, Ya. M.;Koroleva;Eremin;Agabekov [Russian Journal of General Chemistry,2018,vol. 88,# 3,p. 410 - 417][Zh. Obshch. Khim.,2018,vol. 88,# 3,p. 382 - 389,8]
99-65-0
4 suppliers
$32.00/25g

541-69-5
108 suppliers
$34.00/25g