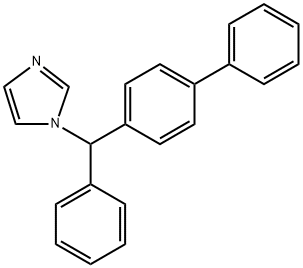
Bifonazole synthesis
- Product Name:Bifonazole
- CAS Number:60628-96-8
- Molecular formula:C22H18N2
- Molecular Weight:310.4
of melting point 142°C are obtained.
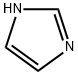
288-32-4
972 suppliers
$5.00/25g
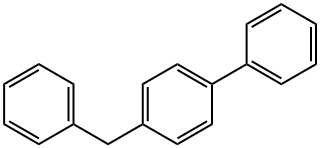
613-42-3
104 suppliers
$8.00/1g

60628-96-8
391 suppliers
$5.00/250mg
Yield:60628-96-8 77%
Reaction Conditions:
Stage #1:4-benzylbiphenyl with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethane for 0.833333 h;Inert atmosphere;Reflux;
Stage #2:1H-imidazole with potassium carbonate in acetonitrile for 1 h;Reflux;
Steps:
89 Example 89
Under a nitrogen atmosphere, a mono-substituted aromatic substrate 8e (6.0 mmol), thioanthracene-S-oxide (7.2 mmol), DCM (30.0 mL) were added to a 25 mL Schlenk tube, followed by stirring at -40°C.After slowly dropping Tf2O (7.2 mmol), it was stirred at -40°C for 30 minutes, followed by stirring at room temperature for 1 hour.Then, under a nitrogen atmosphere, sodium bicarbonate (0.6 mmol), pinacol phenyl borate (9.0 mmol), bis(tri-tert-butylphosphine) palladium (0.3 mmol), DMF (30.0 mL) was added, and the bottle was screwed tightly. Cover and react at 50°C for 12 hours.After the reaction was completed, the celite was filtered, and the solvent was removed under reduced pressure. The crude product was separated and purified by a preparation plate (toluene/hexane (40/1)) to obtain a white solid 11 (1.24 g) with a yield of 84%.Under a nitrogen atmosphere, 11 (0.2 mmol), NBS (0.2 mmol), AIBN (0.02 mmol), and CCl4 (2.5 mL) were added to a 20 mL reaction flask.Reflux for 50 minutes.Cooled to room temperature, the reaction mixture was filtered and washed twice with n-hexane (2×2 mL).After concentration, imidazole (1.57 mmol), potassium carbonate (0.61 mmol) and acetonitrile (4.3 mL) were added.Reflux for 1 hour.After cooling to room temperature, the reaction was quenched by adding a small amount of DCM, filtered through celite, and the solvent was removed under reduced pressure. The crude product was separated and purified by the preparation plate (hexane/EtOAc (5/3)) to obtain the drug molecule 12 (Bifonazole) (47.9mg ), yield 77%.
References:
Chinese Academy Of Sciences Shanghai Organic Chemistry Institute;Wang Peng;Chen Xiaoyue;Nie Xiaoxue;Wu Yichen CN111187130, 2020, A Location in patent:Paragraph 0506-0508; 0510

288-32-4
972 suppliers
$5.00/25g
![alpha-phenyl[1,1'-biphenyl]-4-methanol](/CAS/GIF/7598-80-3.gif)
7598-80-3
42 suppliers
$95.00/1g

60628-96-8
391 suppliers
$5.00/250mg

7515-73-3
10 suppliers
inquiry
18156-74-6
434 suppliers
$8.00/5g

60628-96-8
391 suppliers
$5.00/250mg
![alpha-phenyl[1,1'-biphenyl]-4-methanol](/CAS/GIF/7598-80-3.gif)
7598-80-3
42 suppliers
$95.00/1g

60628-96-8
391 suppliers
$5.00/250mg
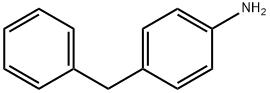
1135-12-2
132 suppliers
$6.00/1g

60628-96-8
391 suppliers
$5.00/250mg