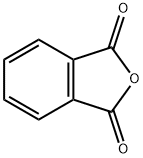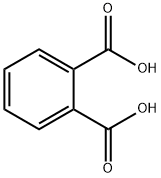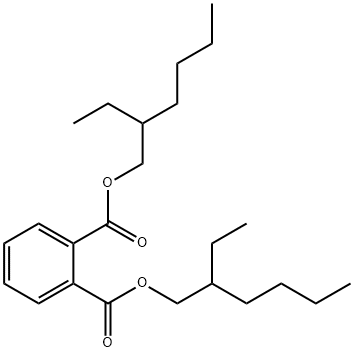
Bis(2-ethylhexyl) phthalate synthesis
- Product Name:Bis(2-ethylhexyl) phthalate
- CAS Number:117-81-7
- Molecular formula:C24H38O4
- Molecular Weight:390.56
Yield:117-81-7 100%
Reaction Conditions:
with 3,3′-(2,2-bis(hydroxymethyl)propane-1,3-diyl)bis(1-methyl-1H-imidazol-3-ium) hydrogen sulfate for 2 h;Dean-Stark;Reflux;
Steps:
2.4. Synthesis of dibutyl phthalate as a typical procedure for esterificationreaction of phthalic anhydride with butanol
General procedure: The typical procedure for esterification of phthalic anhydride withbutanol is as follows: phthalic anhydride (1 mmol, 0.15 g), butanol(5 mmol, 0.46 mL, 0.37 g) and HFDAIL as catalyst (10 mol% to phthalicanhydride, 0.05 g) were charged into a 50 mL round bottom flask witha dean-stark apparatus, reflux condenser and a magnetic stirrer. Thenthe mixture was stirred at 125 °C for 7 h. The completion of reactionwas monitored by TLC using (EtOAC/Hexane 2:8) as eluent. After completionof the reaction as indicated by TLC, the product (dibutyl phthalate)was separated simply by extraction with ethyl acetate (3 × 5 mL)and ethyl acetatewas evaporated under vacuum to afford desired productas yellowoil liquid at 95% yield (0/26 g, boiling point=340 °C). Theobtained product dried under vacuumat 70 °C for 5 h. Viscous ionic liquidcould be reused after removal ofwater under vacuumat 80 °C for 5 hwithout any disposal.
References:
Fareghi-Alamdari, Reza;Nadiri Niri, Mehri;Hazarkhani, Hassan [Journal of Molecular Liquids,2017,vol. 227,p. 153 - 160]
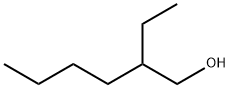
104-76-7
657 suppliers
$5.00/10g

4376-20-9
119 suppliers
$52.00/250mg

117-81-7
688 suppliers
$14.00/25g

104-76-7
657 suppliers
$5.00/10g
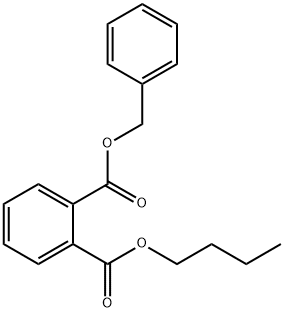
85-68-7
282 suppliers
$27.00/25g

117-81-7
688 suppliers
$14.00/25g
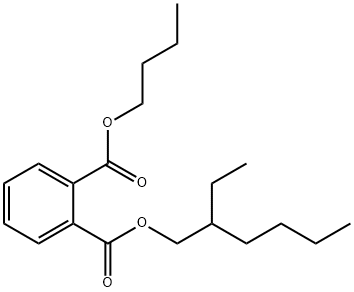
85-69-8
11 suppliers
inquiry