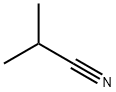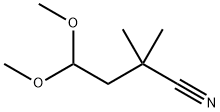
Butanenitrile, 4,4-dimethoxy-2,2-dimethyl- synthesis
- Product Name:Butanenitrile, 4,4-dimethoxy-2,2-dimethyl-
- CAS Number:31004-47-4
- Molecular formula:C8H15NO2
- Molecular Weight:157.21
Yield:31004-47-4 87%
Reaction Conditions:
Stage #1: 4,4-dimethoxybutanenitrilewith lithium diisopropyl amide in tetrahydrofuran;hexane at -10; for 0.5 h;Inert atmosphere;
Stage #2: with lithium diisopropyl amide in tetrahydrofuran;hexane;dichloromethane at -78; for 1 h;Inert atmosphere;
Stage #3: methyl iodidewith lithium diisopropyl amide in tetrahydrofuran;hexane;dichloromethane at -78; for 1 h;Inert atmosphere;
Steps:
6 Example 6: 4,4-Dimethoxy-2,2-dimethylbutanenitrile.
To a solution of diisopropylamine (4.77 mL, 34.1 mmol, 2.2 equiv) in THF (50 mL) at -10°Cunder N2 was added 1.5 M n-BuLi in hexanes (22.7 mL, 34.1 mmol, 2.2 equiv). After 30 mm the mixturewas cooled to -78 °C and a solution of nitrile (2.0 g, 15.5 mmol, 1.0 equiv) in THF (10 mL) was added.After 1 h iodomethane (2.12 mL, 34.1 mmol, 2.2 equiv) was added. The mixture was slowly warmed to 0°C and kept there for 14 h, at which time it was quenched with sat. aq. NH4C1 (40 mL) and extracted withEtOAc (3 x 20 mL). The organics were dried over Na2SO4, filtered and concentrated in vacuo. Theresultant oil was purified by flash chromatography on silica gel (5:1 -3:1 hexanes/EtOAc) to yield theproduct (2.105 g, 87%) as a yellow oil. R1= 0.49 (3:1 hexanes/EtOAc). ‘HNMR(400 MHz, CDC13) 3:4.60 (t, 1H, J= 5.6 Hz), 3.37 (s, 6H), 1.83 (d, 2H, J= 4.4 Hz), 1.39 (s, 6H); ‘3C NMR (100 MHz, CDC13)3: 124.7, 102.4, 53.3, 43.0, 30.0, 27.5
References:
WO2015/187998,2015,A2 Location in patent:Paragraph 00501
14618-78-1
130 suppliers
$9.00/1g

592479-02-2
8 suppliers
$265.00/50mg

74-88-4
341 suppliers
$15.00/10g

31004-47-4
0 suppliers
inquiry