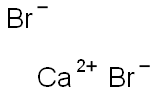
Calcium bromide synthesis
- Product Name:Calcium bromide
- CAS Number:7789-41-5
- Molecular formula:Br2Ca
- Molecular Weight:199.89
CaCO3 (solid)+HBr (liq)→CaBr2 (liq)+ CO2 (gas)
An improved method for producing calcium bromide has been developed by reacting hydrogen bromide with calcium hydroxide in the presence of water.

7440-70-2
139 suppliers
$17.00/5g
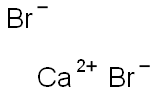
7789-41-5
369 suppliers
$14.00/1g
Yield:-
Reaction Conditions:
with hydrogen bromide in not givenformation of single crystal; product not isolated;
References:
Yamaguchi, S. [Bulletin of the Chemical Society of Japan,1943,vol. 18,p. 53 - 91]